Coulomb's Law is a fundamental principle in physics that calculates the amount of force between two electrically charged particles at rest. It was first published in 1785 by French physicist Charles-Augustin de Coulomb and states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. The law is applicable to point charges, which are smaller than the distance between them. It can also be applied to non-point charges, such as charged spheres or cylinders, if the charges are concentrated in a small enough area.
| Characteristics | Values |
|---|---|
| Scope | Coulomb's Law calculates the amount of force between two electrically charged particles at rest. |
| Charge type | Applies to both attractive and repulsive forces between charged particles. |
| Charge movement | Only applies to static charges. |
| Charge size | Only applies to point charges. |
| Charge distance | The charges must not overlap. |
What You'll Learn
- Coulomb's Law calculates the force between two electrically charged particles at rest
- The law is named after French physicist Charles-Augustin de Coulomb, who first published it in 1785
- Coulomb's Law is used in laser printers, powder coating, and Xerox machines
- The electrostatic force is conventionally called the Coulomb force
- Coulomb's Law is only valid for point and static charges

Coulomb's Law calculates the force between two electrically charged particles at rest
Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. It was first published in 1785 by French physicist Charles-Augustin de Coulomb, and it was essential to the development of the theory of electromagnetism.
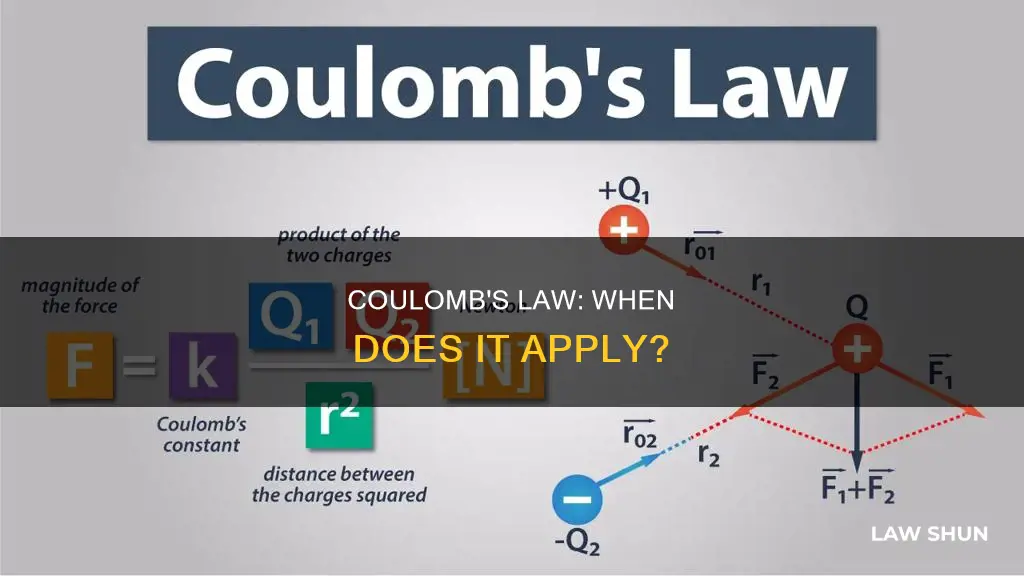
Coulomb's Law states that the magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. The electrostatic force is conventionally called the Coulomb force.
The law can be written as:
|F| = ke * (|q1| * |q2|) / r^2
Here, ke is a constant, q1 and q2 are the quantities of each charge, and the scalar r is the distance between the charges. The force acts along the straight line joining the two charges.
Coulomb's Law is similar to Isaac Newton's inverse-square law of universal gravitation. However, gravitational forces always cause attraction, while electrostatic forces can cause attraction or repulsion. Additionally, gravitational forces are much weaker than electrostatic forces.
Coulomb's Law is valid for point charges and static charges. It does not apply to two charged bodies of finite sizes, as the distribution of charge does not remain uniform when the bodies are brought together. It also does not apply to moving charges, as the information about the position of the charge can only travel at the speed of light.
Coulomb's Law has many applications in modern life, from Xerox machines to laser printers to powder coating. It also holds within atoms, correctly describing the force between the positively charged atomic nucleus and the negatively charged electrons.
The Physics of Other Worlds Explained
You may want to see also

The law is named after French physicist Charles-Augustin de Coulomb, who first published it in 1785
Coulomb's law is named after French physicist Charles-Augustin de Coulomb, who first published it in 1785. De Coulomb was born in Angoulême, Angoumois county, France, in 1736 and died in Paris in 1806. He was an officer, engineer, and physicist. He studied philosophy, language, literature, mathematics, astronomy, chemistry, and botany at Collège Mazarin in Paris.
De Coulomb's formulation of the law that now bears his name was an outgrowth of his attempt to investigate the law of electrical repulsions as stated by Joseph Priestley of England. He invented a device called a torsion balance, which allowed him to measure very small charges and experimentally estimate the force of attraction or repulsion between two charged bodies. The data he obtained through his extensive use of the torsion balance enabled him to formulate one of the fundamental laws of electromagnetism.
Coulomb's law states that the force between two electrical charges is proportional to the product of the charges and inversely proportional to the square of the distance between them. The law is similar to Isaac Newton's inverse-square law of universal gravitation but differs in that gravitational forces always attract, while electrostatic forces can cause attraction or repulsion. Coulomb's law is essential to the development of the theory of electromagnetism and can be used to describe the forces that bind atoms and molecules together to form solids and liquids.
E-Waste Laws: Global Regulations for a Sustainable Future
You may want to see also

Coulomb's Law is used in laser printers, powder coating, and Xerox machines
Coulomb's Law, which describes the behaviour of electrostatic forces, has many applications in modern life, including Xerox machines, laser printers, and powder coating.
Xerox Machines
The electrostatic process is what makes copies in Xerox machines. It involves a selenium-coated aluminium drum, as selenium is an insulator in the dark and a conductor when exposed to light. A negative charge is induced under a thin layer of positively charged selenium. The drum is then exposed to the image to be copied, and the positive charge is neutralised where the image is light, while it remains where the image is dark. A dry black powder called toner is then sprayed with a negative charge, which is attracted to the positive areas of the drum. A blank piece of paper is given a greater positive charge than the drum, allowing it to pull the toner from the drum. Finally, the paper and toner are passed through heated rollers to melt and permanently adhere the toner to the paper.
The force of attraction between unlike charges and the force of repulsion between like charges, as described by Coulomb's Law, is used for copying printed content in Xerox machines.
Laser Printers
Laser printers use a similar process to Xerox machines. A laser beam is scanned across a photoconducting drum, leaving a positively charged image. The rest of the process is the same as in xerography. Laser printers can produce very high-quality images due to the precise control of laser light.
Powder Coating
Powder coating, or electrostatic painting/coating, uses a high-voltage electrostatic charge applied to both the object being coated and the sprayer mechanism. This charge accelerates the coating towards the object, creating a uniform coating that adheres extremely well. Multiple powder colours can be applied before curing them together, allowing for colour blending and bleeding that produces special effects.
Applying for a Law PhD: A Guide
You may want to see also

The electrostatic force is conventionally called the Coulomb force
Coulomb's law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. The electrostatic force is conventionally called the Coulomb force.
Coulomb's law states that the magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. The force is along the straight line joining the two charges. If the charges have the same sign, the electrostatic force between them makes them repel; if they have different signs, the force between them makes them attract.
Coulomb's law is defined as:
F=k * (q1*q2)/r^2
Where F is the force, k is Coulomb's constant, q1 and q2 are the charges on the two particles, and r is the distance between the particles.
Coulomb's law is valid only for point and static charges. It is not valid for moving charges because the information about the position of the charge (the field caused by the charge) can only travel at the speed of light. It also does not apply to two charged bodies of finite sizes, as the distribution of charge does not remain uniform when the two bodies are brought together.
Coulomb's law has many applications in modern life, from Xerox machines to laser printers to powder coating.
Applying to NYU Law: A Comprehensive Guide
You may want to see also

Coulomb's Law is only valid for point and static charges
Coulomb's Law is a fundamental principle in physics that describes the electrostatic interaction between two charged particles. It states that the magnitude of the electrostatic force between two point charges is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Coulomb's Law is valid only for point charges and static charges. The electrostatic force between two point charges can be calculated using the equation referring to the distance from the source, which is only defined for a point, not a distribution.
For large bodies, one needs to integrate the partial forces exerted on differential volumes of the bodies. However, for uniformly charged spheres or spherical shells, Coulomb's Law can be used by inserting the distance between the centres of the spheres in the formula.
Coulomb's Law does not apply to two charged bodies of finite sizes, as the distribution of charge does not remain uniform when the two bodies are brought together.
Coulomb's Law is also not valid for moving charges. This is because the information about the position of the charge (the field caused by the charge) can only travel at the speed of light.
Good Samaritan Laws: Doctors and Legal Protection
You may want to see also







