Gas laws are a group of laws that describe the behaviour of gases, specifically in relation to pressure, volume, and temperature. One such law is Dalton's Law, also known as Dalton's Law of Partial Pressures, which states that the pressure of a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. This law is only applicable to ideal gases, and in reality, most gases will not follow it exactly, especially under conditions of extremely high pressure. Another law that applies to mixtures of gases is Amagat's Law, or the Law of Partial Volumes, which describes the behaviour and properties of mixtures of ideal gases, and in some cases, non-ideal gases.
| Characteristics | Values |
|---|---|
| Name | Dalton's Law (Law of Partial Pressures) |
| Date of Observation | 1801 |
| Date of Publication | 1802 |
| Observer | John Dalton |
| Formula | P_total = P_1 + P_2 + P_3 + ... + P_n |
| Formula (with partial pressures) | p_total = p_1 + p_2 + p_3 + ... + p_n |
| Formula (with mole fraction) | p_i = p_total * x_i |
| Formula (with volume-based concentration) | p_i = p_total * c_i |
| Applicability | Only applies to ideal gases |
What You'll Learn

Dalton's Law of Partial Pressure
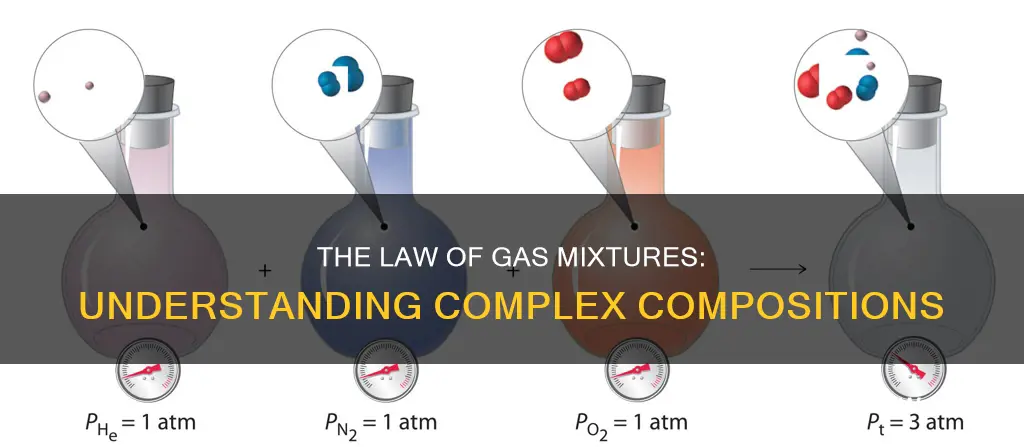
Dalton's Law, also known as the Law of Partial Pressures, was observed by John Dalton in 1801 and published in 1802. This law applies only to mixtures of gases and states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture. In other words, each gas exerts the same pressure on a container as it would if it were alone in that container.
Mathematically, the pressure of a mixture of non-reactive gases can be defined as:
\\[P_{total} = \sum_{i=1}^{n}p_{i} = p_{1} + p_{2} + p_{3} + ... + p_{n}\]
Where p1, p2, ..., pn represent the partial pressures of each component.
The mole fraction of a specific gas in a mixture of gases is equal to the ratio of the partial pressure of that gas to the total pressure exerted by the gaseous mixture. This mole fraction can also be used to calculate the total number of moles of a constituent gas when the total number of moles in the mixture is known.
Dalton's Law is related to the ideal gas laws. Real gases do not strictly follow Dalton's Law, with the deviation increasing with pressure. Under high-pressure conditions, the volume occupied by the molecules becomes significant compared to the free space between them.
Human Rights Law: Does It Protect Terrorists?
You may want to see also

Amagat's Law of Partial Volume
Amagat's Law, also known as the Law of Partial Volumes, describes the behaviour and properties of mixtures of ideal gases and, in some cases, non-ideal gases. It is named after Emile Amagat, a French physicist who published his law of partial volumes in 1880.
The law relates the total volume of a mixture with the volumes of individual components. It states that the total volume of a non-reacting mixture of gases at a constant temperature and pressure is equal to the sum of the individual partial volumes of the constituent gases. This can be expressed mathematically as:
> {\displaystyle V=V_{1}+V_{2}+V_{3}+\dots +V_{n}=\sum _{i}V_{i}.}
Amagat's Law assumes that the volumes of the component gases are additive, meaning that at the same temperature and pressure, the interactions of the different gases are the same as the average interactions of the components. This can be interpreted in terms of a second virial coefficient for the mixture.
Amagat's Law is very similar to Dalton's Law of Partial Pressures, which states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. However, while Dalton's Law assumes that the gases in the mixture are non-interacting, Amagat's Law assumes that the gases interact and that these interactions are the same as the average interactions of the components.
Amagat's Law is of use in chemistry and thermodynamics. It can be used to approximate the behaviour of real gases, although it is most accurate for ideal gases.
Lemon Law: Private Sellers and You
You may want to see also

Avogadro's Law
The law can be written as:
${\displaystyle V\propto n}$
${\displaystyle {\frac {V}{n}}=k}$
Where V is the volume of the gas, n is the amount of substance of the gas (measured in moles), and k is a constant for a given temperature and pressure.
The volume occupied by one gram-mole of gas is about 22.4 litres at standard temperature and pressure (0°C, 1 atmosphere) and is the same for all gases, according to Avogadro's Law. This is known as the Avogadro constant.
AI Fairness: Do Lending Laws Apply?
You may want to see also

Henry's Law
The law can be stated as:
> At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Mathematically, this can be expressed as:
> p = k_H*c
Where p is the partial pressure of the solute in the gas above the solution, c is the concentration of the solute, k_H is the Henry's Law Constant, and the solubility of the substance is k.
Another example is in the production of carbonated beverages. Carbonated drinks contain dissolved carbon dioxide gas, which is under high pressure and at a pressure greater than atmospheric pressure. When the container is opened, the pressure decreases, and the solubility of the carbon dioxide decreases, causing it to form bubbles and escape from the liquid.
Understanding the Legal Requirements for Applying for Medicare
You may want to see also

Boyle's Law
The law can be expressed mathematically as:
P ∝ (1/V)
Or
P x V = k
Where P is the pressure of the gas, V is the volume of the gas, and k is a constant for a particular temperature and amount of gas.
Understanding Photo Copyright Laws and Their Applications
You may want to see also







