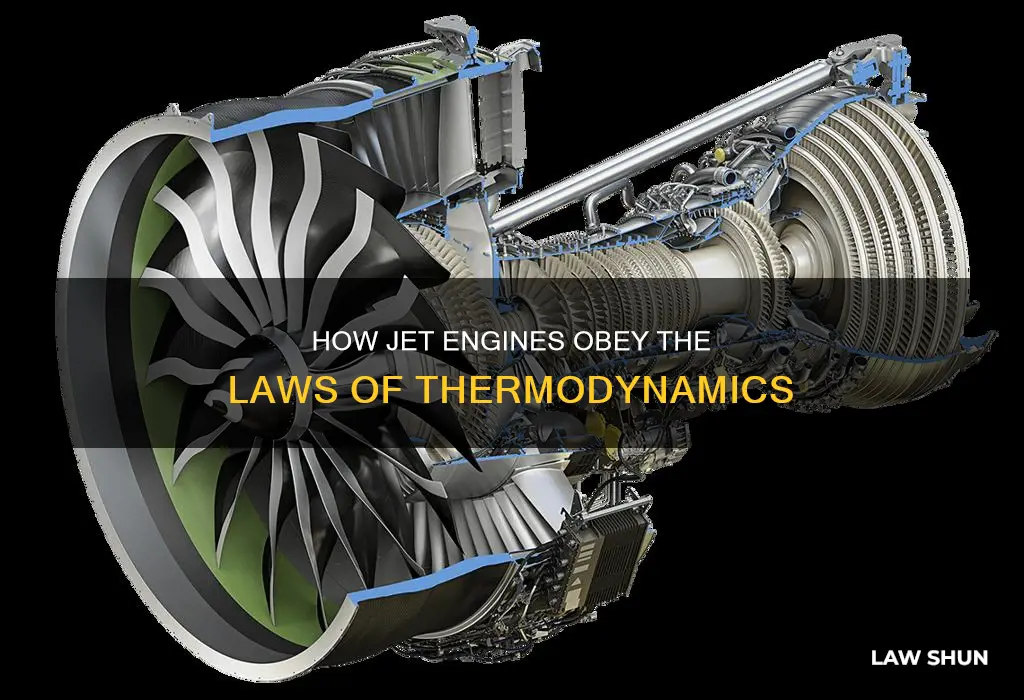
Jet engines are a prime example of thermodynamics in action. As a type of heat engine, jet engines are governed by the laws of thermodynamics, which dictate the movement and transformation of heat and energy in mechanical systems. Understanding these laws is crucial for engineers looking to improve engine efficiency and performance. By applying the principles of energy conservation and entropy, engineers can design more sustainable and efficient jet engines. The first law of thermodynamics, also known as the law of energy conservation, is particularly relevant to jet engines as it underpins the conversion of chemical energy from fuel into mechanical energy. This law states that energy cannot be created or destroyed in an isolated system, only transformed from one form to another. The second law, which introduces the concept of entropy, is also critical to understanding jet engine efficiency. It states that the total entropy of an isolated system will always increase over time, meaning that some energy will always be lost as waste heat. This has important implications for jet engine design, as engineers must create systems that minimise energy loss and maximise the conversion of heat into useful work.
What You'll Learn

Jet engines are a type of heat engine
The compressor is powered by a turbine, which extracts energy from the expanding gas passing through it. The turbine is attached to the same shaft as the compressor, and spinning the turbine causes the compressor to spin. The engine converts internal energy in the fuel to increased momentum of the gas flowing through the engine, producing thrust.
The Brayton thermodynamic cycle, also known as the Joule cycle, is a common way to describe the functioning of jet engines. It is a cycle that involves four processes: compression, heat addition, expansion, and heat rejection. The air flowing into the jet engine is compressed, then heated by adding fuel and igniting the mixture. This heated, compressed air then expands and the resulting exhaust gases are expelled, pushing the engine forward.
The efficiency of jet engines is controlled primarily by the operating conditions inside the engine, such as the pressure produced by the compressor and the temperature of the combustion gases. The efficiency is also influenced by how smoothly the air and combustion gases flow through the engine and how well the flow is aligned with the moving and stationary passages in the compressors and turbines. Additionally, the complex and dynamic nature of jet engines makes it challenging to accurately model and predict their behavior under various conditions.
Overall, jet engines are a type of heat engine that operates on the Brayton thermodynamic cycle, and their efficiency is influenced by various factors related to their internal components and airflow.
Lease-Option Transactions: Confirmation Laws Applicable?
You may want to see also

The Brayton cycle and jet propulsion
The Brayton cycle, also known as the Joule cycle, is a thermodynamic cycle that describes the operation of certain heat engines that use air or another gas as their working fluid. It is characterised by isentropic compression and expansion, and isobaric heat addition and rejection. The cycle is named after George Brayton, who developed the Brayton Ready Motor in 1872, using a piston compressor and piston expander.
The Brayton cycle is commonly used in jet engines and gas turbine engines. In a simple gas turbine engine arrangement, air is drawn into a compressor, where the pressure increases. The compressor output airstream then enters a combustor, where fuel is injected and combustion takes place. The heated combustion products then enter a gas turbine and expand, producing work. The Brayton cycle models this cycle through isentropic compression, constant-pressure heat addition, isentropic expansion, and constant-pressure heat rejection.
The Brayton cycle can be represented on pressure-volume and temperature-entropy diagrams. The thermal efficiency of the cycle is influenced by the pressure ratio and specific-heat ratio. The addition of a heat exchanger or regenerator can further improve the cycle's efficiency by extracting energy from the exhaust stream and preheating the inlet air.
The Brayton cycle is a continuous-combustion device, with separate regions of the engine serving single thermodynamic functions. This is in contrast to intermittent-combustion cycles, where all thermodynamic processes occur in a piston-cylinder device. The Brayton cycle is an idealised representation of the properties of a fixed mass of gas as it passes through a gas turbine in operation.
The Brayton cycle is a power conversion system that uses a gas to produce thrust in jet engines or power in natural gas turbines. It achieves this by compressing and heating air, then expanding it through a turbine to generate power. The cycle can be enhanced with heat exchangers and other components to improve its performance.
When Drugs Are Involved, Do Dram Shop Laws Apply?
You may want to see also

The Carnot cycle and jet engine design
The Carnot cycle is a fundamental thermodynamic cycle that serves as a theoretical framework for understanding the operation of engines. It was first proposed by Nicolas Leonard Sadi Carnot in 1824 and involves four successive operations: isothermal expansion, adiabatic expansion, isothermal compression, and adiabatic compression. This cycle helps determine the maximum possible efficiency of a heat engine during the conversion of heat into work.
The Carnot cycle is particularly significant in jet engine design. Jet engines are complex systems that involve the intake, compression, combustion, and expulsion of air and fuel. The cycle helps engineers understand the underlying thermodynamics and make informed design decisions. By applying the Carnot cycle, engineers can determine the maximum possible efficiency of a jet engine and identify areas for improvement. This is crucial for optimizing the engine's performance at different altitudes and speeds, which are key considerations for aircraft propulsion systems.
One of the key challenges in jet engine design is managing high temperatures and pressures. The Carnot cycle assists in this aspect by providing insights into the relationship between temperature, pressure, and volume. By understanding the idealized behavior of gases within the cycle, engineers can design systems that minimize energy losses and maximize efficiency. The cycle's reversible nature, where the gas can return to its original state, is particularly useful for understanding the potential for energy conversion within the engine.
Additionally, the Carnot cycle helps engineers analyze the trade-offs between engine efficiency and power requirements. While the Carnot cycle represents the most efficient way to convert heat into work, practical jet engines often deviate from this ideal cycle due to factors such as power-to-weight ratios and specific power requirements. Engineers must balance the theoretical optimal efficiency of the Carnot cycle with the practical considerations of jet engine operation.
In summary, the Carnot cycle is a fundamental tool in jet engine design. It provides a theoretical framework for understanding the efficiency of heat engines and guides engineers in optimizing the performance of jet engines. By applying the principles of the Carnot cycle, engineers can make informed decisions to improve engine efficiency, manage high temperatures and pressures, and balance efficiency with power requirements.
Antitrust Laws: Individual Accountability and Legal Boundaries
You may want to see also

Applying the First Law of Thermodynamics to jet engines
The First Law of Thermodynamics, also known as the law of energy conservation, is based on experimental observations. It states that the total amount of energy within a system remains constant, even though it can be transformed from one form to another. This means that the amount of heat transferred into a system, plus the amount of work done on the system, must result in a corresponding increase in the system's internal energy.
Jet engines are a type of heat engine, which is a device that uses heat to produce work or uses work to move heat around. Other examples of heat engines include refrigerators and internal combustion engines. In the case of jet engines, they take in air through an intake, compress it, mix it with fuel, and ignite it in a combustion chamber. This process increases the internal energy of the system, in accordance with the First Law of Thermodynamics.
The First Law can be applied to jet engines through ideal cycle analysis, which involves modelling heat engines as thermodynamic cycles. This analysis provides estimates of thermal efficiency and work output as a function of pressures and temperatures at various points in the cycle. While these estimates represent the best achievable performance, the actual performance of jet engines may be lower due to factors such as energy losses and operating conditions.
The thermodynamic process of a typical jet engine can be modelled using an ideal Brayton cycle. This cycle consists of three main processes: isentropic compression, where air is pressurized; isobaric combustion, where heat is added through the addition of fuel; and isentropic expansion, where the hot gases expand through a turbine to produce work. By understanding and optimizing these processes, engineers can improve the efficiency and performance of jet engines.
Overall, the First Law of Thermodynamics provides a fundamental framework for understanding and improving jet engine performance. By analyzing the heat and work transfers within the system, engineers can design more efficient engines that maximize the conversion of fuel and air into useful work, propelling aircraft forward.
Labor Laws and Churches: California's Unique Religious Exemption
You may want to see also

Applying the Second Law of Thermodynamics to jet engines
The second law of thermodynamics deals with the direction taken by spontaneous processes. Many processes occur spontaneously in one direction only, meaning they are irreversible under a given set of conditions. For example, heat transfer occurs spontaneously from higher- to lower-temperature bodies but never spontaneously in the reverse direction. This is the basis of our first version of the second law of thermodynamics.
Now, let's consider how this law applies to jet engines. A jet engine works by taking in air through an intake, compressing it, mixing it with fuel, and igniting it in a combustion chamber. The hot exhaust gases then expand through a turbine, which powers the compressor and the jet's fan, propelling the aircraft forward.
The second law of thermodynamics states that it is impossible in any system for heat transfer from a reservoir to completely convert to work in a cyclical process where the system returns to its initial state. In other words, we can never get more work out of a system than the heat energy we put into it. This has important implications for jet engine design and efficiency.
The efficiency of a jet engine is defined as the ratio of the useful work output to the energy input. In the context of a jet engine, this means the ratio of the thrust produced to the heat energy generated during combustion. According to the second law of thermodynamics, this efficiency can never be 100%, as there will always be some heat transfer to the environment or other losses.
Engineers can, however, work to maximize the efficiency of jet engines by minimizing energy losses. This can be achieved through careful design and optimization of the engine's components, such as the compressor, combustor, and turbine. Additionally, the choice of fuel and operating conditions, such as altitude and speed, can also impact the engine's efficiency.
Overall, the second law of thermodynamics provides a fundamental understanding of the limitations and challenges of jet engine design and helps engineers make informed decisions to improve engine performance.
Truancy Laws in PA: Do They Apply to 18-Year-Olds?
You may want to see also
Frequently asked questions
Yes, the laws of thermodynamics are pivotal in understanding how jet engines work and are applied to their design and functioning.
The first law of thermodynamics, often referred to as the law of energy conservation, states that energy cannot be created or destroyed in an isolated system. In jet engines, this law underpins the conversion of chemical energy from fuel into mechanical energy, which propels the aircraft forward.
The second law of thermodynamics introduces the concept of entropy, stating that the total entropy of an isolated system will always increase over time. This means that some energy will always be lost as waste heat in a jet engine, making it impossible to achieve 100% efficiency.
The Carnot cycle is a theoretical cycle that describes the most efficient way to convert heat into work. It helps engineers determine the maximum possible efficiency of a jet engine and guides their efforts to improve its efficiency.
Some challenges include managing high temperatures and pressures, minimising energy losses, and optimising the engine's performance across different altitudes and speeds. The complex nature of jet engines also makes accurate modelling and prediction of their behaviour difficult.







