The laws of thermodynamics are a set of scientific laws that define a group of physical quantities, such as temperature, energy, and entropy, which characterise thermodynamic systems in thermodynamic equilibrium. There are four laws of thermodynamics: the first law, the second law, the third law, and the zeroth law. The laws of thermodynamics govern the transfer of energy in and among all systems in the universe. The first law of thermodynamics states that the total amount of energy in the universe is constant; energy can be transformed from one form to another, but it cannot be created or destroyed. The second law of thermodynamics states that every energy transfer involves some loss of energy in an unusable form, such as heat energy, resulting in a more disordered system. The third law of thermodynamics states that a system's entropy approaches a constant value as the temperature approaches absolute zero. The zeroth law of thermodynamics defines thermal equilibrium and forms the basis for the definition of temperature.
| Characteristics | Values |
|---|---|
| First Law of Thermodynamics | Energy cannot be created or destroyed |
| The total amount of energy in the universe is constant | |
| Energy can be transferred between the system and the surroundings | |
| Energy can be transferred or transformed | |
| Second Law of Thermodynamics | For a spontaneous process, the entropy of the universe increases |
| For a spontaneous process, ΔSuniverse > 0 | |
| For a spontaneous process, ΔSsystem + ΔSsurroundings > 0 | |
| Entropy constantly increases in a closed system | |
| Every energy transfer involves some loss of energy in an unusable form | |
| Third Law of Thermodynamics | A perfect crystal at zero Kelvin has zero entropy |
| The entropy of an isolated system approaches a constant value as the temperature of the system approaches absolute zero | |
| Zeroth Law of Thermodynamics | If two bodies are each in thermal equilibrium with a third body, they must also be in equilibrium with each other |
What You'll Learn

The First Law of Thermodynamics
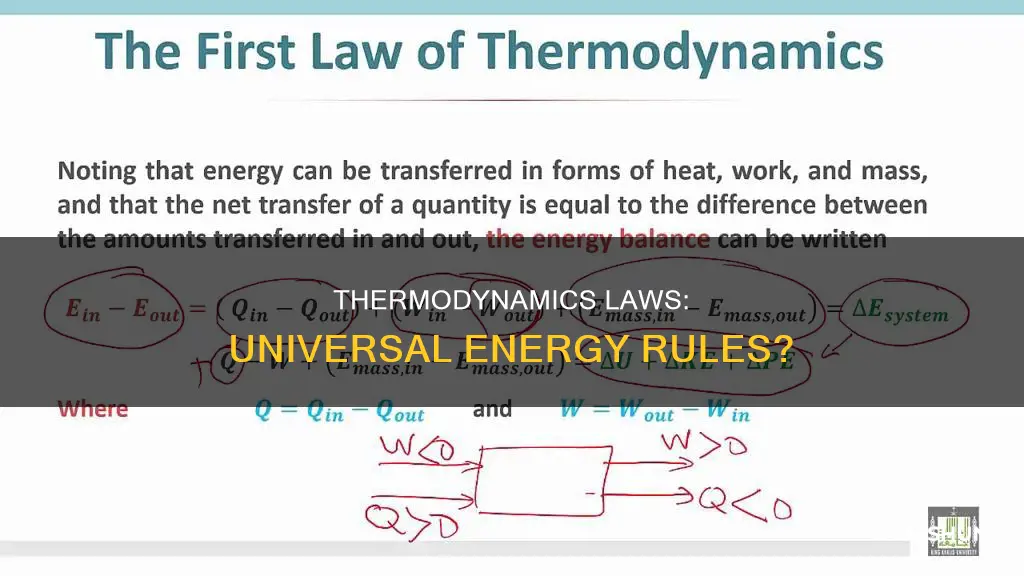
The First Law distinguishes two principal forms of energy transfer—heat and thermodynamic work—that modify a thermodynamic system containing a constant amount of matter. It also defines the internal energy of a system, which is an extensive property used to account for the balance of heat and work in the system. The internal energy of a system is a state variable, like temperature or pressure, and is dependent only on the state of the system and not on any process.
The First Law encompasses several principles, including the conservation of energy, which states that energy can be neither created nor destroyed, but can only change form. It also includes the concept of internal energy and its relationship to temperature. The internal energy of a system is the sum of its kinetic energy, potential energy, and internal energy. Work is a process of transferring energy to or from a system in ways that can be described by macroscopic mechanical forces acting between the system and its surroundings.
Antitrust Laws: Microsoft's Friend or Foe?
You may want to see also

The Second Law of Thermodynamics
- For a spontaneous process, the entropy of the universe increases.
- For a spontaneous process, ΔSuniverse > 0.
- For a spontaneous process, ΔSsystem + ΔSsurroundings > 0.
The second law was formulated by Rudolf Clausius in the 1850s and included his statement that heat can never pass from a colder to a warmer body without some other change occurring at the same time. The second law may also be expressed as: "Not all heat can be converted into work in a cyclic process."
The second law is concerned with the direction of natural processes. It asserts that a natural process runs only in one sense and is not reversible. That is, the state of a natural system itself can be reversed, but not without increasing the entropy of the system's surroundings.
The second law indicates the irreversibility of natural processes and, in many cases, the tendency of natural processes to lead towards spatial homogeneity of matter and energy, especially of temperature. It can be formulated in a variety of interesting and important ways. One of the simplest is the Clausius statement, that heat does not spontaneously pass from a colder to a hotter body.
The second law also allows the definition of the concept of thermodynamic temperature, but this has been formally delegated to the zeroth law of thermodynamics.
Lemon Laws: Medical Equipment Exempt or Included?
You may want to see also

The Third Law of Thermodynamics
The Third Law was developed by German chemist Walther Nernst between 1906 and 1912 and is, therefore, often referred to as the Nernst heat theorem. It is sometimes called the Nernst-Simon heat theorem to acknowledge the contribution of Nernst's doctoral student, Francis Simon.
The Third Law is not intuitive but was derived empirically as a system's entropy always approached the same minimum value as the absolute temperature was lowered and approached zero. It provides a reference point that, combined with the fact that entropy is a state function, allows us to determine the absolute entropy of a substance at any temperature. This is very useful for calculating the change in entropy during a reaction.
The Third Law can be applied to perfect crystals, which have achieved thermodynamic equilibrium and are in a crystalline state where all atoms, ions, and molecules are in well-defined positions in a highly ordered crystalline lattice. This excludes amorphous solids like glass that don't have an ordered, crystalline structure and have not achieved thermodynamic equilibrium.
Mathematically, the absolute entropy of any system at zero temperature is the natural log of the number of ground states times Boltzmann's constant (kB).
Vagrancy Laws: Southern Whites and Their Exemptions
You may want to see also

Energy Transformation
The laws of thermodynamics govern the transfer of energy in all systems in the universe. The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed or transferred. This means that the total amount of energy in the universe has always been and always will be the same. Energy can be moved from place to place and changed into different forms, but the overall amount of energy remains constant.
The second law of thermodynamics states that every energy transfer involves some loss of energy in an unusable form, usually heat energy, resulting in a more disordered system. In other words, no energy transfer is completely efficient. For example, when an airplane flies, some of the energy is lost as heat energy due to friction with the air. Similarly, during cellular metabolic reactions, energy is lost as heat. This is beneficial for warm-blooded creatures as it helps maintain body temperature.
The third law of thermodynamics states that a system's entropy approaches a constant value as its temperature approaches absolute zero. In practical terms, this makes reaching absolute zero impossible as extracting energy from a system becomes increasingly difficult as its temperature lowers.
The first law of thermodynamics can be applied to energy transformation by understanding that energy can be transferred or transformed, but the total amount of energy in a closed system remains constant. For instance, turning on a light switch transforms electrical energy into light energy, and a gas stove transforms chemical energy from natural gas into heat energy.
The second law of thermodynamics also applies to energy transformation by highlighting that all energy transfers and transformations result in some loss of usable energy. For example, cooking on a stove results in a loss of heat energy to the surrounding air, and driving a car leads to energy loss as heat energy due to friction with the pavement.
The third law of thermodynamics is less directly applicable to everyday energy transformations but is a crucial aspect of the broader energy landscape. It provides insight into the behaviour of systems as they approach absolute zero, which has implications for various scientific and industrial processes.
Lemon Law: Private Sellers and You
You may want to see also

Energy Conservation
The First Law of Thermodynamics, also known as the Law of Conservation of Energy, has far-reaching implications. It means that any gain in energy by a system corresponds to a loss in energy by its surroundings, and vice versa. This law applies to both open and closed systems. An open system allows for the transfer of energy and matter between the system and its surroundings, while a closed system does not permit such transfers.
In a closed system, the First Law dictates that the change in the system's internal energy is equal to the difference between the heat added to the system and the work done by the system on its surroundings. This principle holds true even when there is a transfer of matter involved. For example, when two isolated systems are combined, the total internal energy of the new system is equal to the sum of the internal energies of the initial systems.
The First Law of Thermodynamics also extends beyond the boundaries of individual systems. It asserts that the total energy of the universe has always been and will always be the same. Energy can be transferred or transformed, but never truly lost or created. This concept is crucial in understanding the fundamental nature of energy and how it operates within the universe.
The First Law serves as a foundation for understanding energy dynamics and is applicable across various scientific disciplines, including physics, chemistry, and biology. It provides a basis for exploring the transformations and transfers of energy that occur constantly around us. For instance, light bulbs transform electrical energy into light energy, and gas stoves convert natural gas into heat energy for cooking.
In biological systems, the First Law takes on a unique significance. Living organisms are open systems that constantly exchange energy with their surroundings. They obtain energy from their environment and transform it into usable energy to perform various tasks, such as building complex molecules, powering cellular motion, and reproducing. This process of energy conversion is essential for sustaining life and maintaining the highly ordered state of living systems.
Understanding Lemon Laws: Do They Cover Computers?
You may want to see also