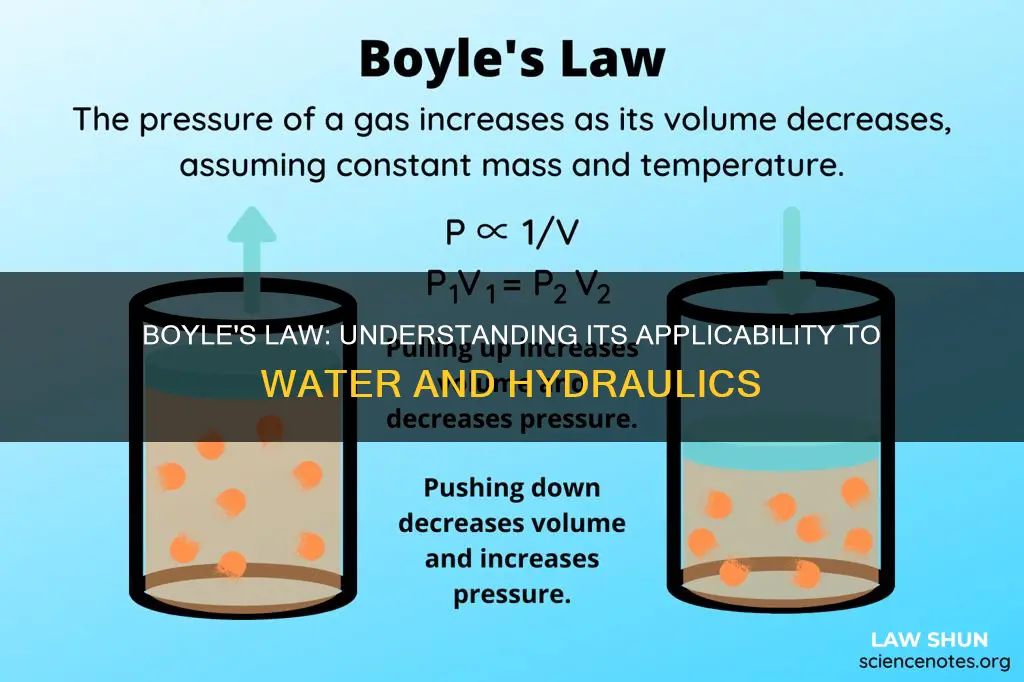
Boyle's Law, also known as the Boyle-Mariotte law, is a fundamental law in chemistry that describes the behaviour of a gas held at a constant temperature. The law was discovered by Robert Boyle in 1662 and states that the volume of a gas is inversely proportional to the pressure exerted by it. In other words, when a gas is pumped into an enclosed space, its volume will decrease, but the pressure it exerts on its container will increase. This law applies to gases and not liquids, as liquids are not compressible. So, does Boyle's Law apply to water?
What You'll Learn

Boyle's Law and scuba diving
Boyle's Law, discovered by Anglo-Irish chemist Robert Boyle in 1662, is a gas law that states that the pressure exerted by a gas is inversely proportional to the volume it occupies, as long as the temperature and the quantity of gas remain constant. The law can be expressed mathematically as P = k*(1/V) ⇒ PV = k, where P is the pressure exerted by the gas, V is the volume occupied by it, and k is a constant.
Boyle's Law is highly relevant to scuba diving, as it helps divers understand the behaviour of air during a dive and explains many of the safety guidelines that must be followed. As a diver descends, the water pressure around them increases, causing the air in their scuba equipment and body to compress and occupy a smaller volume, according to Boyle's Law. Conversely, as a diver ascends, the water pressure decreases, and the air in their gear and body expands to occupy a greater volume.
This compression and expansion of air due to changes in water pressure lead to several important safety considerations in scuba diving. For example, divers must equalise the pressure in their ears during descent to avoid pain and potential injury, known as ear barotrauma. Additionally, they must release excess air from their buoyancy compensator device (BCD) during ascent, as the expanding air can cause a loss of control over buoyancy.
One of the critical safety rules in scuba diving derived from Boyle's Law is the instruction to never hold your breath underwater. If a diver ascends while holding their breath, the air trapped in their lungs will expand, which can stretch the lungs and lead to pulmonary barotrauma. Similarly, ascending too quickly can result in decompression sickness, as the nitrogen gas absorbed by the body during the dive expands and forms tiny bubbles in the blood and tissue.
Boyle's Law enables scuba divers to predict how air volume and pressure will change during a dive and underscores the importance of maintaining a constant temperature to ensure the validity of the equation.
Vagrancy Laws: Southern Whites and Their Exemptions
You may want to see also

The working of a syringe
Boyle's Law, also known as the Boyle-Mariotte Law, is a gas law formulated by Anglo-Irish chemist Robert Boyle in 1662. It states that the pressure exerted by a gas is inversely proportional to the volume it occupies, given that the temperature and amount of gas remain constant. In other words, as the volume of a gas increases, its pressure decreases, and vice versa. This relationship can be expressed mathematically as P ∝ (1/V), where P is the pressure and V is the volume.
Now, to answer your question about the workings of a syringe:
A syringe is a simple reciprocating pump that consists of a plunger (or piston) that fits tightly into a cylindrical tube called a barrel. The plunger can be pulled and pushed along the inside of the tube, allowing the syringe to draw in or expel liquid or gas through an orifice at the front, open end of the tube. The open end of the syringe may be fitted with a hypodermic needle, nozzle, or tubing to direct the flow of the liquid or gas. Syringes are frequently used in clinical medicine to administer injections, infuse intravenous therapy, apply compounds such as glue or lubricant, and draw or measure liquids.
The barrel of a syringe is usually made of plastic or glass and has graduated marks to indicate the volume of fluid inside. The barrel is almost always transparent. Plastic syringes can be designed with two or three parts. A three-part syringe has a plastic plunger/piston with a rubber tip to create a seal between the piston and the barrel, while a two-part syringe creates a perfect fit between the plastic plunger and the barrel without the need for a separate rubber tip. Most modern medical syringes are made of plastic as they are cheap and disposable, reducing the risk of spreading blood-borne diseases.
Syringes are also used in dentistry to inject anaesthetics and in veterinary medicine to irrigate wounds or large abscesses. Additionally, they have non-medical applications, such as injecting gravy into meats, manufacturing candies, and refilling ink cartridges in fountain pens.
Livestream Legalities: Do Wiretapping Laws Apply?
You may want to see also

The operation of human lungs
Boyle's law is a gas law that states that the pressure exerted by a gas of a given mass, kept at a constant temperature, is inversely proportional to the volume occupied by it. In other words, the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant.
Now, onto the operation of human lungs.
The lungs are a pair of spongy, pinkish-grey organs in the chest and are the centerpiece of the respiratory system. The respiratory system's main job is to transport oxygen into the body and remove carbon dioxide, a waste product. The body needs oxygen for energy, which it gets from the air around us.
When you breathe in through your nose or mouth, you pull air into your throat and down the trachea (windpipe). The trachea then divides into two bronchial tubes, one for each lung, which further split into smaller tubes called bronchioles. At the end of each bronchiole are alveoli, tiny air sacs where the exchange of oxygen and carbon dioxide takes place.
Oxygen from the air you breathe in moves from the alveoli into the bloodstream through tiny blood vessels called capillaries. Red blood cells then pick up the oxygen and deliver it to the body's cells. At the same time, carbon dioxide, a waste gas, moves from the blood into the lungs to be exhaled. This process is called gas exchange and is essential to life.
The diaphragm, a dome-shaped muscle, is the primary muscle responsible for breathing. It does this by expanding and contracting the chest to draw air in and out of the lungs. The rib muscles also help by pulling the ribs upward and outward during inhalation, making the chest cavity bigger, and relaxing during exhalation, causing the chest cavity to get smaller.
In addition to gas exchange, the respiratory system also plays a role in maintaining the proper body temperature and humidity level of inhaled air, protecting the body from harmful substances, and supporting the sense of smell.
Vaping vs Smoking: Are Vaping Laws Different?
You may want to see also

Why do bottles fizz when opened?
You may have experienced opening a bottle of soda and having the liquid fizz up and out of the bottle. This phenomenon is caused by the carbon dioxide gas that is added to the liquid to make it fizzy. When the bottle is sealed, the carbon dioxide is under pressure and is dissolved in the liquid. Opening the bottle releases the pressure, and the gas comes out of the liquid, forming bubbles. This is an example of Boyle's law in action.
Boyle's law, discovered by Robert Boyle in 1662, is a gas law that describes the relationship between the pressure and volume of a confined gas. The law states that at a constant temperature, the volume of a gas is inversely proportional to the pressure exerted by the gas. In other words, when the volume of a gas increases, the pressure decreases, and vice versa.
In the case of a carbonated beverage, the carbon dioxide gas is dissolved in the liquid under pressure. When the bottle is opened, the volume of the container increases, and according to Boyle's law, the pressure inside the bottle decreases. This decrease in pressure causes the carbon dioxide gas to come out of the solution, forming bubbles.
The same principle can be observed when pumping air into a bicycle tire. As you pump air into the tire, the gas molecules inside the tire get compressed and packed closer together, increasing the pressure. This increase in pressure makes the tire feel tight and firm.
Boyle's law also explains the operation of our lungs when we breathe. When we inhale, our diaphragm lowers, increasing the volume inside our lungs. This decrease in volume leads to a decrease in pressure, causing air to be drawn into our lungs. When we exhale, our diaphragm pushes upwards, reducing the volume inside our lungs and increasing the pressure, forcing the air out.
In summary, Boyle's law states that the volume and pressure of a gas are inversely proportional to each other at a constant temperature. This law explains various phenomena, including the fizzing of bottles when opened, the behaviour of bicycle tires, and the mechanics of breathing.
Laws on Reservations: Who Has Jurisdiction?
You may want to see also

Why did flight attendants' skirts feel tight at cruising altitude?
Boyle's law, also known as the Boyle-Mariotte law, is a fundamental principle in chemistry that describes the behaviour of gas held at a constant temperature. The law was formulated by Anglo-Irish chemist Robert Boyle in 1662 and states that the pressure exerted by a gas is inversely proportional to the volume it occupies, provided the temperature and amount of gas remain the same. In other words, as the volume of a gas increases, its pressure decreases, and vice versa.
Now, let's delve into the mystery of why flight attendants' skirts felt tight at cruising altitude. This intriguing phenomenon can be explained using Boyle's Law. When an aircraft ascends to a higher altitude, the cabin pressure decreases, leading to a reduction in pressure inside the flight attendants' stomachs. As a result, according to Boyle's Law, the volume of their stomachs increases, causing their stomachs to bulge. This increase in stomach volume can make the uniforms they wore on the ground feel tighter at cruising altitude.
To address this issue, airlines like British Airways introduced adjustable skirts for their female flight attendants. This adjustment ensured that their uniforms remained comfortable and well-fitted, even when facing changes in air pressure during flights.
Boyle's Law has practical applications beyond this tight-skirt mystery. It helps explain various everyday phenomena, such as the operation of a syringe, the working of our lungs during inhalation and exhalation, and the behaviour of gases in scuba diving equipment.
Insider Trading Laws: Private Companies and Legal Boundaries
You may want to see also
Frequently asked questions
No, Boyle's Law only applies to gases. It describes the inverse relationship between the pressure exerted by a gas and the volume it occupies, provided the temperature and amount of gas remain constant.
Boyle's Law helps explain the effects of changing water pressure on a diver's body and equipment. As a diver descends, the increased water pressure causes the air in their body and scuba gear to compress and occupy a smaller volume. Conversely, as a diver ascends, the decreased water pressure leads to the expansion of air in their gear and body, requiring the release of excess air to maintain control.
One example is the fizz that escapes when opening a bottle of soda. The carbon dioxide gas added to the liquid is under built-up pressure, and when the bottle is opened, the gas-liquid mixture rushes out due to the increased volume and decreased pressure. Another example is the human respiratory system, where inhalation and exhalation increase and decrease the volume of the chest cavity, respectively, leading to changes in air pressure within the lungs.
The equation for Boyle's Law is PV = k, where P represents the pressure of the gas, V is the volume of the gas, and k is a constant value representing the temperature and amount of gas.