The ideal gas law, also known as the general gas equation, is a hypothetical equation of state for an ideal gas. It is a good approximation for the behaviour of many gases under various conditions, though it has certain limitations. The ideal gas law combines Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It relates the four independent properties of a gas at any given time: pressure, volume, temperature, and the amount of substance. The ideal gas law is often used to solve for mass and can be applied to molar volumes, density, and stoichiometry problems. However, it is important to note that the ideal gas law assumes no intermolecular forces and zero atomic volume, making it a purely hypothetical concept.
| Characteristics | Values |
|---|---|
| The ideal gas law relates the four independent physical properties of a gas at any time | Pressure, Volume, Temperature, Amount |
| The ideal gas law can be used in stoichiometry problems in which chemical reactions involve gases | |
| Standard temperature and pressure (STP) are a useful set of benchmark conditions to compare other properties of gases | 100 kPa for pressure and 273 K for temperature |
| At STP, gases have a volume of 22.4 L per mole | |
| The ideal gas law can be used to determine densities of gases |
What You'll Learn
- The ideal gas law can be used to calculate the volume of a gas
- It can also be used to calculate the number of moles of gas present
- The ideal gas law can be used to calculate the temperature of a gas
- It can be used to calculate the pressure of a gas
- The ideal gas law can be used to calculate the density of a gas

The ideal gas law can be used to calculate the volume of a gas
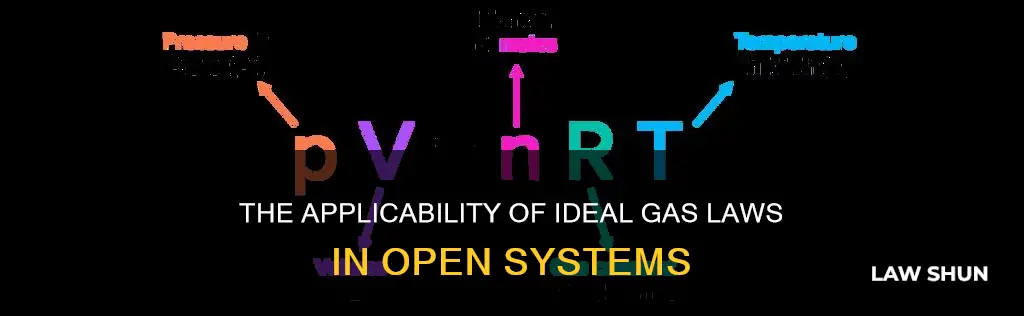
> pV = nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the amount of substance of gas (also known as the number of moles)
- R is the ideal or universal gas constant
- T is the absolute temperature of the gas
> V = nRT / p
For example, let's say we want to find the volume of 40 moles of a gas at a pressure of 1013 hPa and a temperature of 250 K. We can plug these values into the formula:
> V = (40 moles) * (8.31446261815324) * (250 K) / (101300 Pa)
This gives us a volume of 0.82 m³.
It's important to note that the ideal gas law assumes certain conditions, such as low density to prevent the emergence of strong intermolecular forces. The gas constant, R, also depends on the units used in the calculation. Additionally, the ideal gas law may not be applicable to all types of gases or conditions, and there are other gas laws that may be more suitable in certain situations.
Child Labor Laws: Emancipated Minors' Rights Explained
You may want to see also

It can also be used to calculate the number of moles of gas present
The ideal gas law, also called the general gas equation, is an equation of state of a hypothetical ideal gas. It can be used to calculate the number of moles of gas present in a given volume. The ideal gas law is often written in the following empirical form:
PV = nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the amount of substance of gas (also known as the number of moles)
- R is the ideal, or universal, gas constant
- T is the absolute temperature of the gas
To calculate the number of moles of gas present, we can rearrange the ideal gas law equation to the following:
N = (pV) / (RT)
Where p, V, and T are measured in pascals, cubic metres, and kelvins, respectively. R has a value of 8.314 J/(mol·K) = 1.989 ≈ 2 cal/(mol·K), or 0.0821 L⋅atm/(mol⋅K).
For example, let's say we have a gas with a pressure of 7.00 × 10^5 Pa, a volume of 2.00 × 10^-3 m^3, and a temperature of 18.0ºC (or 291 K). We can plug these values into our equation to calculate the number of moles:
N = (7.00 × 10^5 Pa × 2.00 × 10^-3 m^3) / (8.314 J/(mol·K) × 291 K)
N = 0.579 mol
So, there are 0.579 moles of gas present in this example.
It's important to note that the ideal gas law assumes certain conditions, such as a large number of molecules that move around randomly and no intermolecular forces, in order for it to accurately describe the behaviour of a gas.
America's Jewish Population: Miscegenation Law Effects
You may want to see also

The ideal gas law can be used to calculate the temperature of a gas
To calculate the temperature of a gas, follow these steps:
- Ensure that the values for pressure, volume, and amount of substance are consistent units. For example, use pascals for pressure and cubic meters for volume.
- Calculate the product of pressure and volume.
- Calculate the product of the number of moles and the gas constant.
- Divide the result of step 2 by the result of step 3. The result is the temperature of the gas in Kelvin.
It is important to note that the ideal gas law assumes ideal conditions, such as negligible intermolecular forces and no interactions between gas molecules except for collisions. In reality, gases may deviate from ideal behaviour at extremely high or low temperatures and pressures.
US Laws and Non-Citizens: Who's Affected and How?
You may want to see also

It can be used to calculate the pressure of a gas
The ideal gas law, also known as the general gas equation, is a good approximation of the behaviour of many gases under various conditions. It can be used to calculate the pressure of a gas, along with its other properties, such as volume, temperature, and amount.
The ideal gas law is expressed as:
PV = nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the amount of substance of gas (also known as the number of moles)
- R is the ideal or universal gas constant
- T is the absolute temperature of the gas
By rearranging the equation, we can solve for pressure:
P = nRT/V
This formula allows us to calculate the pressure of a gas when we know its volume, temperature, and amount. For example, let's consider a sample of gas with a volume of 3.91 L, a temperature of 305 K, and an amount of 2.09 moles. By plugging these values into the equation, we can calculate the pressure as follows:
P = (2.09 moles)(0.082057 L atm/mol K)(305 K)/3.91 L
P = 1.45 atm
Thus, the pressure of the gas is 1.45 atmospheres.
It's important to note that the ideal gas law assumes ideal gas behaviour, which may not always hold true for real gases. However, it is a useful approximation and can provide valuable insights into the behaviour of gases.
Thermodynamics Laws: Universal or Not?
You may want to see also

The ideal gas law can be used to calculate the density of a gas
The ideal gas law, also known as the general gas equation, is an equation of state for a hypothetical ideal gas. It was first stated by Benoît Paul Émile Clapeyron in 1834 and has since been used to approximate the behaviour of many gases under various conditions.
The ideal gas law is often written in the following form:
PV=nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the amount of substance of gas (or number of moles)
- R is the ideal, or universal, gas constant
- T is the absolute temperature of the gas
The ideal gas law can also be used to calculate the density of a gas. To do this, we can rearrange the equation to substitute the number of moles (n) with mass (m) and volume (V) to get:
PV=mRT
Where:
M is the mass of the gas
We can then substitute the mass (m) and volume (V) with the density of the gas (ρ) to get:
P=ρRT
This formula allows us to calculate the density of a gas using its pressure, temperature, and the ideal gas constant. The density of a gas can vary significantly, even among ideal gases. For example, hydrogen has a much lower density than butane, with values of 0.07927 kg/m³ and 2.281 kg/m³, respectively.
It is important to note that the ideal gas law is an idealisation, and real gases may deviate from it, especially as temperature and pressure approach the critical point. In such cases, a correction factor called the "compressibility factor" can be used to quantify the deviation from ideal gas behaviour.
Fair Housing Laws: Who Do They Protect in Georgia?
You may want to see also
Frequently asked questions
The ideal gas law is an equation that describes the behaviour of an ideal gas, which is a hypothetical gas that follows certain assumptions, such as having no intermolecular forces and zero volume. The equation is often written as PV = nRT, where P is pressure, V is volume, n is the amount of substance, and T is temperature.
The ideal gas constant, R, depends on the units used for pressure and volume. Different values of R are given in the literature, such as 0.082057 L atm mol-1 K-1, 62.364 L Torr mol-1 K-1, or 8.3145 m3 Pa mol-1 K-1. Choose the value of R that matches the units of your given information.
Yes, the ideal gas law can be applied to open systems, which allow mass flow across the system boundary. When using the ideal gas law for open systems, ensure that you account for any mass flow rates and choose the appropriate value for the ideal gas constant based on your units.
The ideal gas law is a good approximation for many gases under various conditions, but it has limitations. It assumes ideal gas behaviour, which neglects molecular size and intermolecular attractions. Therefore, it is most accurate for monatomic gases at high temperatures and low pressures. More detailed equations, such as the van der Waals equation, account for deviations from ideality.







