The Law of Multiple Proportions, also known as Dalton's Law, is a fundamental rule of chemistry. It states that when two elements react to form several compounds, the ratio of their masses that mix with a fixed mass of the other element is in the ratio of tiny whole numbers. This law was proposed by John Dalton in 1803 and later formed the basis of stoichiometry. Ionic bonding, on the other hand, is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or atoms with different electronegativities. It is one of the main types of bonding, along with covalent and metallic bonding. So, does the Law of Multiple Proportions apply to ionic bonds?
What You'll Learn

How does the law of multiple proportions relate to ionic bonds?
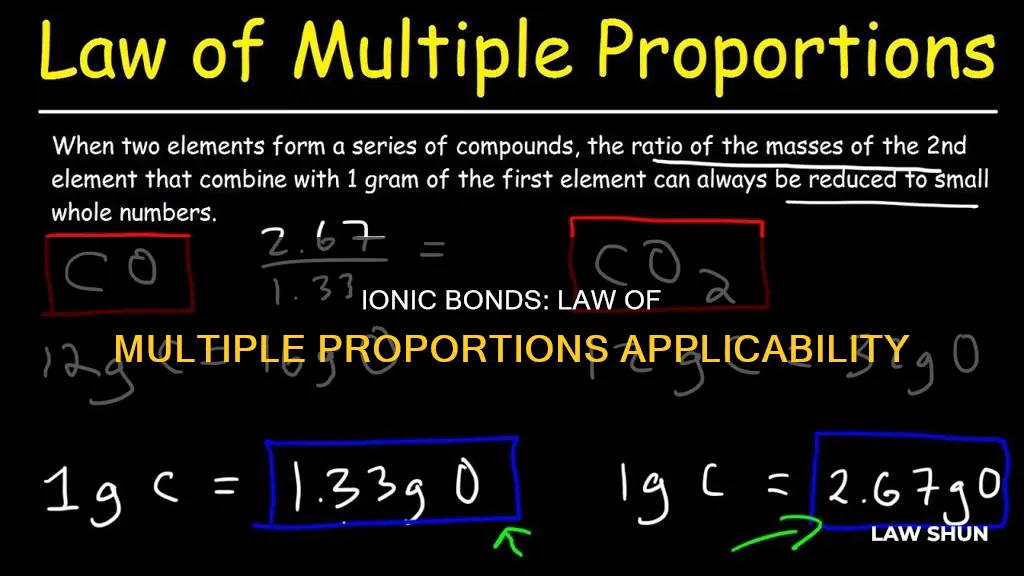
The law of multiple proportions, also known as Dalton's Law, states that when two elements form more than one compound, the mass ratios of the second element that combines with a fixed mass of the first element will always be the ratios of small whole numbers.
This law was proposed by John Dalton in 1803 and published in his book, 'New System of Chemical Philosophy' (Vol 1). It is based on the earlier work of French chemist Joseph Proust, who proposed the law of definite proportions, which states that elements combine in certain well-defined proportions.
The law of multiple proportions is particularly relevant to ionic bonding, a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities. Ionic bonding is one of the main types of bonding, along with covalent and metallic bonding.
In ionic bonding, atoms transfer electrons to achieve a full valence shell, forming positively charged ions (cations) and negatively charged ions (anions). The transfer of electrons results in a stable electron configuration for both atoms. The cation is typically a metal atom, while the anion is usually a non-metal atom.
The law of multiple proportions applies to ionic bonding because it describes the ratios in which elements combine to form compounds. For example, in the formation of common table salt, sodium chloride (NaCl), sodium atoms lose an electron to form cations (Na+), while chlorine atoms gain an electron to form anions (Cl-). These ions then combine in a 1:1 ratio to form NaCl.
The law of multiple proportions helps to explain the formation of ionic compounds and their stoichiometry, which refers to the quantitative relationships between reactants and products in chemical reactions. It is a fundamental concept in chemistry that provides insights into the behaviour and reactivity of chemicals.
Case Law: Retrospective Application and Its Implications
You may want to see also

What are ionic bonds?
Ionic bonding is a type of chemical bond that occurs between two oppositely charged ions. It involves the complete transfer of valence electrons between atoms, as opposed to covalent bonding, where electrons are shared. Ionic bonds are formed when a metallic atom reacts with a non-metallic atom. This is due to the high difference in electronegativities between the two atoms.
Ionic bonding occurs when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that loses the electrons becomes a positively charged ion (cation), and the atom that gains them becomes a negatively charged ion (anion). These ions are arranged so that the positive and negative charges alternate and balance each other out, resulting in an overall neutral charge.
An example of an ionic compound is sodium chloride (NaCl). Sodium (Na) donates one of its electrons to chlorine (Cl) in a chemical reaction. The resulting positive ion (Na+) and negative ion (Cl-) form a stable ionic compound.
Ionic bonds typically form when the difference in electronegativities between the two atoms is large. In contrast, covalent bonds are more likely to form when the electronegativities are similar. Ionic bonding can be considered an extreme case of a polar covalent bond, which results from unequal electron sharing rather than complete electron transfer.
Gravity's Laws: Do They Apply Everywhere?
You may want to see also

How does the law of multiple proportions apply to ionic compounds?
The law of multiple proportions, also known as Dalton's Law, states that when two elements form more than one compound, the mass ratios of the second element that combines with a fixed mass of the first element will always be the ratios of small whole numbers. This law was proposed by John Dalton in 1803.
Ionic bonding is a type of chemical bonding that involves the electrostatic attraction between oppositely charged ions or between two atoms with sharply different electronegativities. It is one of the main types of bonding, along with covalent and metallic bonding. Ions are atoms or groups of atoms with an electrostatic charge. Atoms that gain electrons form negatively charged ions (anions), while atoms that lose electrons form positively charged ions (cations).
The law of multiple proportions applies to ionic compounds in the following way:
When two elements form more than one ionic compound, the ratio of the masses of the second element that combines with a fixed mass of the first element will be in the ratio of small whole numbers. This is because ionic compounds, in general, obey the rules of stoichiometry, which states that strict ratios between anions and cations are observed to maintain charge neutrality.
For example, let's consider the formation of two ionic compounds: sodium chloride (NaCl) and magnesium chloride (MgCl2). In NaCl, one sodium atom (Na) combines with one chlorine atom (Cl) to form the compound. In MgCl2, one magnesium atom (Mg) combines with two chlorine atoms (Cl). The ratio of the masses of chlorine that combines with a fixed mass of sodium or magnesium will be 1:2, which is a ratio of small whole numbers.
Another example is the formation of aluminum oxide (Al2O3) and aluminum oxide (AlO). In Al2O3, two aluminum atoms (Al) combine with three oxygen atoms (O). In AlO, one aluminum atom combines with one oxygen atom. The ratio of the masses of oxygen that combines with a fixed mass of aluminum is 3:1, which is again a ratio of small whole numbers.
Therefore, the law of multiple proportions applies to ionic compounds by considering the ratios of the masses of the elements involved in the formation of multiple compounds.
Kepler's Third Law: Beyond Our Sun's Reach?
You may want to see also

How does the law of multiple proportions relate to stoichiometry?
The law of multiple proportions and stoichiometry are closely related concepts in chemistry. Stoichiometry is the branch of chemistry that deals with the quantitative relationships between the reactants and products in a chemical reaction. It involves determining the amounts of substances involved in a reaction, often expressed in terms of moles or mass. The law of multiple proportions, on the other hand, specifically addresses the ratios in which elements combine to form compounds.
The law of multiple proportions, also known as Dalton's Law, was formulated by John Dalton in 1803. It states that when two elements combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element will be in a ratio of small whole numbers. In other words, if elements A and B form two compounds, AB and AB2, the mass of element A in AB will be half the mass of A in AB2 for a given mass of B. This law is a fundamental concept in chemistry and was instrumental in the development of stoichiometry and our understanding of chemical reactions.
The law of multiple proportions provides a basis for stoichiometry by establishing the quantitative relationships between elements in different compounds. For example, let's consider the reaction between hydrogen and oxygen to form water:
> 2H2 + O2 → 2H2O
In this reaction, two moles of hydrogen gas (H2) react with one mole of oxygen gas (O2) to produce two moles of water (H2O). The law of multiple proportions helps us understand that the ratio between hydrogen and oxygen in water is 2:1. This ratio is crucial for stoichiometric calculations, as it allows us to determine the amount of reactants needed or products formed in a reaction.
Furthermore, the law of multiple proportions also applies to ionic compounds, which involve the transfer of electrons between atoms to form ions. Ionic compounds, such as sodium chloride (NaCl), are held together by electrostatic forces between oppositely charged ions. While the ratios between ions in ionic compounds may not always be whole numbers, they still follow the rules of stoichiometry. For example, in NaCl, the ratio between sodium (Na+) and chloride (Cl-) ions is always 1:1, maintaining stoichiometric proportions.
In summary, the law of multiple proportions and stoichiometry are interconnected concepts. The law of multiple proportions provides a foundation for stoichiometry by establishing the ratios in which elements combine to form compounds, whether covalent or ionic. Stoichiometry then utilizes these ratios to determine the quantitative relationships between reactants and products in chemical reactions, helping chemists understand and predict the outcomes of these reactions.
International Waters: Navigating Complex Legal Waters
You may want to see also

What are the limitations of the law of multiple proportions?
The law of multiple proportions is a fundamental rule in chemistry. It states that when two elements react to form several compounds, the ratio of their masses that mix with a fixed mass of the other element is in the ratio of small whole numbers. However, this law has some limitations.
Firstly, the law is best demonstrated using simple compounds. It often does not apply when comparing very large molecules. For example, when comparing the hydrocarbons decane (C10H22) and undecane (C11H24), the ratio of hydrogen masses is approximately 121:120, which deviates from the expected small whole number ratio.
Secondly, the law fails with compounds of the non-stoichiometric type and does not work well with oligomers and polymers. Stoichiometry refers to the quantitative relationships between reactants and products in chemical reactions, and non-stoichiometric compounds do not follow these precise ratios.
Additionally, the law assumes that elements combine in simple, whole-number ratios. However, this is not always the case, especially in complex organic molecules, where the constituent elements may not exhibit simple ratios.
Furthermore, the law of multiple proportions is a general rule and may not account for exceptions or unique cases in chemistry. It is essential to recognize that while this law provides a valuable framework for understanding chemical reactions, it may not encompass all the intricacies and variations that exist in the field of chemistry.
Lastly, the law of multiple proportions is closely related to the concept of ionic bonding, which involves the electrostatic attraction between oppositely charged ions or atoms with sharp differences in electronegativity. While ionic bonding is one of the main types of bonding, it is important to note that it is not the only type, and other forms of bonding, such as covalent and metallic bonding, may exhibit different behaviours that are not explained by the law of multiple proportions.
Understanding Crummey Laws: Revocable Trusts and Their Exemptions
You may want to see also







