Raoult's Law, established by French chemist François-Marie Raoult in 1887, is a principle in physical chemistry that describes the ideal behaviour of a solution. It states that the partial vapour pressure of a component in a solution is equal to the vapour pressure of the pure component multiplied by its mole fraction in the solution. Raoult's Law assumes that the intermolecular forces between different molecules and similar molecules are equal, and that their molar volumes are the same. This is analogous to the ideal gas law, which assumes ideal behaviour when the interactive forces between molecules approach zero. However, Raoult's Law is only valid for ideal solutions, which rarely occur in reality due to differences in the uniformity of attractive forces between liquids.
| Characteristics | Values |
|---|---|
| Definition | Raoult's Law states that the vapour pressure of a solvent in a solution (or mixture) is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. |
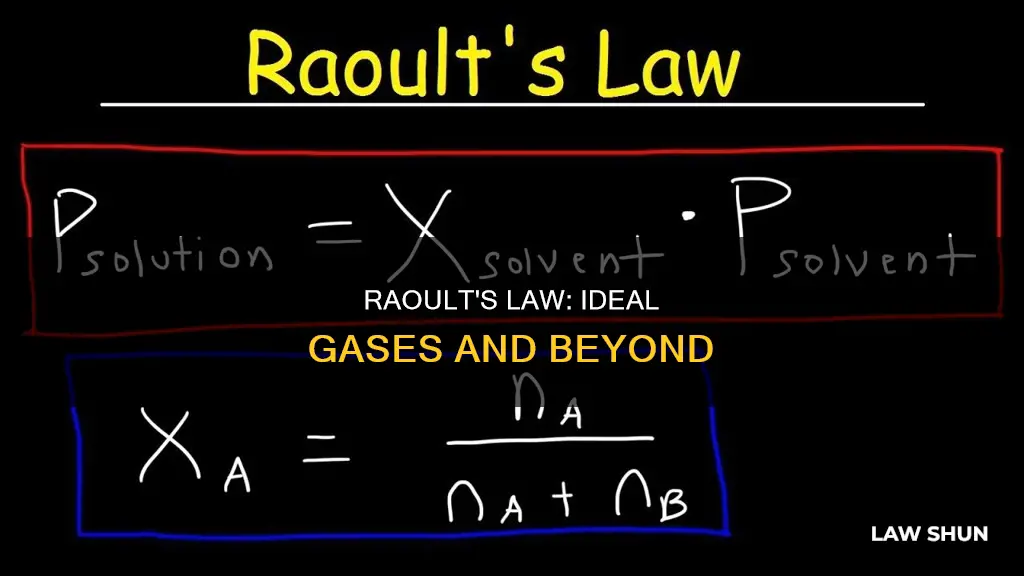
| Formula | Psolution = ΧsolventP0solvent |
| Application | Raoult's Law is useful in calculating the molecular mass of an unknown solute. |
| Similarity to the Ideal Gas Law | Raoult's Law is similar to the ideal gas law, except it applies to solutions. Raoult's Law assumes that the intermolecular forces between different molecules and similar molecules are equal. |
| Applicability | Raoult's Law is only valid for ideal solutions, which are rare. |
| Deviations | There are two types of deviations from Raoult's Law: negative and positive. A negative deviation occurs when the vapour pressure is lower than expected, and a positive deviation occurs when the vapour pressure is higher than expected. |
What You'll Learn

Raoult's Law and the Ideal Gas Law
Raoult's Law, established in 1887 by French chemist François-Marie Raoult, is a relation of physical chemistry with implications in thermodynamics. It states that the partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Mathematically, this can be expressed as:
> Psolution = ΧsolventP0solvent
Where:
- Psolution is the vapour pressure of the solution
- Χsolvent is the mole fraction of the solvent
- P0solvent is the vapour pressure of the pure solvent
Raoult's Law is similar to the ideal gas law, which assumes ideal behaviour in which the intermolecular forces between dissimilar molecules are zero or non-existent. However, Raoult's Law assumes that the intermolecular forces between different molecules and similar molecules are equal. It is a phenomenological relation that assumes ideal behaviour based on the simple microscopic assumption that intermolecular forces between unlike molecules are equal to those between similar molecules, and that their molar volumes are the same.
Raoult's Law only applies to ideal solutions, which rarely occur in nature. An ideal solution is one in which the liquid phase obeys Raoult's Law and the vapour phase obeys the ideal gas law. In reality, many liquids in a mixture do not have the same uniformity in terms of attractive forces, and these types of solutions tend to deviate from the law. There can be either a negative or positive deviation from the law. A negative deviation occurs when the vapour pressure is lower than expected, and it happens when the forces between particles are stronger than those between particles in pure liquids. On the other hand, positive deviation takes place when the cohesion between similar molecules is greater than the adhesion between dissimilar molecules, resulting in both components of the mixture easily escaping from the solution.
American Laws on Reservations: An Indian Jurisdiction
You may want to see also

Raoult's Law and Partial Pressure
Raoult's Law, established by French chemist François-Marie Raoult in 1887, is a principle in physical chemistry that describes the ideal behaviour of a solution by relating the partial vapour pressure of a component to its pure vapour pressure. It states that the vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, the partial vapour pressure of each component of an ideal mixture of liquids is equal to the vapour pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture.
Mathematically, Raoult's Law can be expressed as:
> Psolution = ΧsolventP0solvent
Where:
- Psolution is the vapour pressure of the solution
- Χsolvent is the mole fraction of the solvent
- P0solvent is the vapour pressure of the pure solvent
Raoult's Law is akin to the ideal gas law, except that it relates to the properties of a solution. It assumes that the physical properties of the components of a chemical solution are identical. It also assumes that the molecules of all components in the solution are of comparable size. This means that the intermolecular forces between different molecules and similar molecules are equal.
Raoult's Law only applies to ideal solutions, which are rarely encountered. In reality, many liquids in a mixture do not have the same uniformity in terms of attractive forces, and these types of solutions tend to deviate from the law. There can be either a negative or a positive deviation from the law. A negative deviation occurs when the vapour pressure is lower than expected, and this happens when the forces between particles are stronger than those between particles in pure liquids. An example of negative deviation is a mixture of chloroform and acetone, where hydrogen bonds cause the deviation. On the other hand, positive deviation takes place when the cohesion between similar molecules is greater than the adhesion between dissimilar molecules, resulting in a higher-than-expected vapour pressure. Both components of the mixture can easily escape from the solution. An example of positive deviation includes mixtures of benzene and methanol.
Lease-Option Transactions: Confirmation Laws Applicable?
You may want to see also

Raoult's Law and Mole Fraction
Raoult's Law, a principle of physical chemistry, states that the partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture. It was proposed by French chemist François-Marie Raoult in 1887.
Raoult's Law is defined as:
> The partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Mathematically, this is written as:
> pi = pi* xi
Where:
- Pi is the partial pressure of the component 'i' in the gaseous mixture above the solution
- Pi is the equilibrium vapour pressure of the pure component 'i'
- Xi is the mole fraction of the component 'i' in the liquid or solid solution
Raoult's Law only applies to ideal mixtures. In an ideal mixture, the tendency of the different sorts of molecules to escape is unchanged. This means that the intermolecular forces between two red molecules, two blue molecules, or a red and a blue molecule must all be exactly the same for the mixture to be ideal.
Raoult's Law can be applied to non-ideal solutions, but this requires incorporating two factors that account for the interactions between molecules of different substances. The first factor is a correction for gas non-ideality, or deviations from the ideal gas law, called the fugacity coefficient. The second, the activity coefficient, is a correction for interactions in the liquid phase between the different molecules.
Raoult's Law is similar to the ideal gas law, except that it applies to solutions. It assumes that the intermolecular forces between different molecules and similar molecules are equal, whereas the ideal gas law assumes that the intermolecular forces between dissimilar molecules are zero or non-existent.
Service Dog Laws: Global Application and Access
You may want to see also

Raoult's Law and Volatility
Raoult's Law, named after French chemist François-Marie Raoult, is a principle in physical chemistry that describes the ideal behaviour of a solution by relating the partial vapour pressure of a component to its pure vapour pressure. It states that the vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, the partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Raoult's Law is akin to the ideal gas law, but it applies to solutions. The ideal gas law assumes that the intermolecular forces between dissimilar molecules are zero or non-existent, whereas Raoult's Law assumes that the intermolecular forces between different molecules and similar molecules are equal. Raoult's Law also assumes that the molecules of all components in the solution are of comparable size.
Raoult's Law is valid only for ideal solutions, which are rarely encountered. In reality, the decrease in vapour pressure is greater than that calculated by Raoult's Law for extremely dilute solutions. An ideal solution is one where the solvent-solute interaction is the same as the solvent-solvent or solute-solute interaction. This implies that the solute and solvent take the same amount of energy to escape to the vapour phase as when they are in their pure states. However, ideal solutions are hard to find as different chemical components have to be chemically identical.
Raoult's Law can be applied to non-ideal solutions, but this requires incorporating several factors that consider the interactions between molecules of different substances.
Deviations from Raoult's Law occur when there are adhesive or cohesive forces between two liquids. A negative deviation takes place when the vapour pressure is lower than expected, which happens when forces between particles are stronger than those between particles in pure liquids. An example of a negative deviation is a mixture of chloroform and acetone. Conversely, a positive deviation occurs when the cohesion between similar molecules is greater than the adhesion between dissimilar molecules. This results in a higher-than-expected vapour pressure, as both components of the mixture can easily escape from the solution. An example of a positive deviation is a mixture of benzene and methanol.
Animal Cruelty Laws: Do They Include Fish?
You may want to see also

Raoult's Law and Deviations
Raoult's Law, established by French chemist François-Marie Raoult in 1887, is a relation of physical chemistry with implications in thermodynamics. The law states that the partial vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, the vapour pressure of a solution is dependent on the mole fraction of a solute added to the solution.
Raoult's Law is akin to the ideal gas law, except that it relates to the properties of a solution. The ideal gas law assumes ideal behaviour in which the intermolecular forces between dissimilar molecules are zero or non-existent. On the other hand, Raoult's Law assumes that the intermolecular forces that exist between different molecules and similar molecules are equal.
Raoult's Law only works for ideal solutions, which are rarely encountered. In reality, many liquids in a mixture do not have the same uniformity in terms of attractive forces, and these types of solutions tend to deviate from the law. There are two types of deviations: negative and positive.
A negative deviation occurs when the vapour pressure is lower than expected from Raoult's Law. This happens when the forces between particles are stronger than those between particles in pure liquids. For example, a mixture of chloroform and acetone exhibits a negative deviation from Raoult's Law due to the presence of hydrogen bonds. Another example of a negative deviation is a solution of hydrochloric acid and water.
A positive deviation occurs when the cohesion between similar molecules is greater than the adhesion between dissimilar molecules. This results in a higher-than-expected vapour pressure. Both components of the mixture can easily escape from the solution. Examples of positive deviation include the mixtures of benzene and methanol, and chloroform and ethanol.
Usury Laws: Do They Affect Real Estate Transactions?
You may want to see also
Frequently asked questions
No, Raoult's Law applies to ideal solutions. It states that the partial vapour pressure of a component in a mixture is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
Raoult's Law is a relation of physical chemistry, with implications in thermodynamics. It was proposed by French chemist François-Marie Raoult in 1887.
Raoult's Law helps determine the vapour pressure of a solution, which can be used to calculate the molecular mass of an unknown solute. It also has applications in distillation.
Raoult's Law is meant for ideal solutions, which are rare. It does not account for deviations that occur due to differences in the uniformity of attractive forces between liquids in a mixture.
Both Raoult's Law and the ideal gas law assume ideal behaviour. The ideal gas law assumes that intermolecular forces between dissimilar molecules are zero, while Raoult's Law assumes that intermolecular forces between different molecules and similar molecules are equal.