The second law of thermodynamics is a physical law based on universal empirical observation concerning heat and energy interconversions. It establishes the concept of entropy as a physical property of a thermodynamic system. The law predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics.
The second law of thermodynamics applies to the Earth's atmosphere. For example, the law explains why an ice cube left at room temperature begins to melt. It also explains why the Earth's atmosphere is at a certain temperature.
| Characteristics | Values |
|---|---|
| The Second Law of Thermodynamics | The state of entropy of the entire universe, as an isolated system, will always increase over time. |
| The changes in the entropy in the universe can never be negative. | |
| The second law also states that the net sum of the energy flows will be from hot to cold. |
What You'll Learn

The Second Law of Thermodynamics and the Earth's temperature
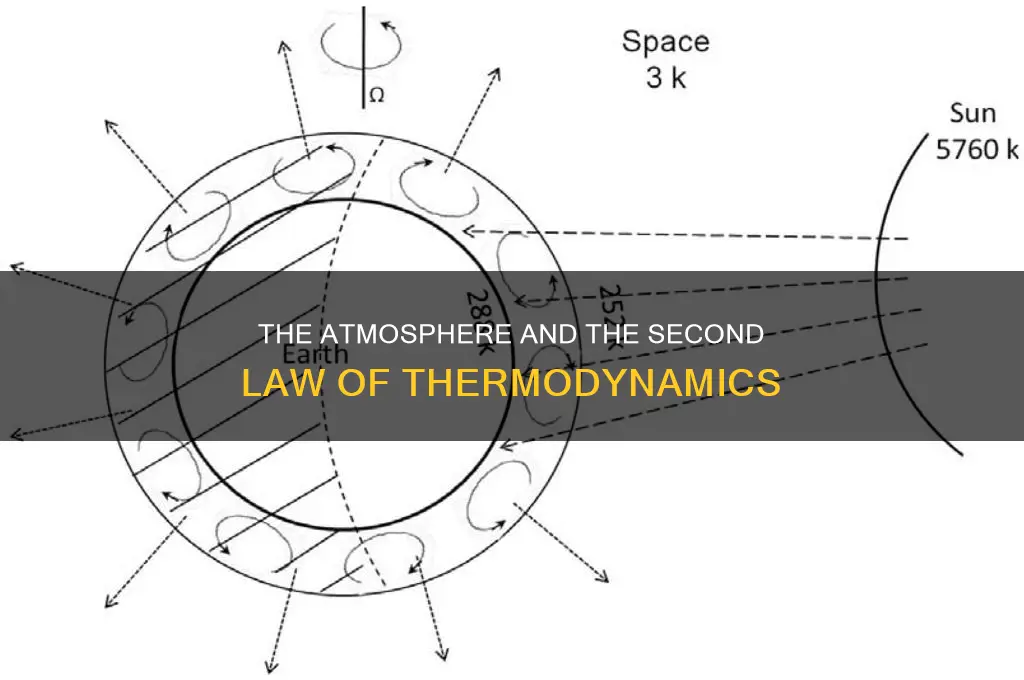
The Second Law of Thermodynamics states that the net flow of energy in an isolated system will always be from hot to cold. This is often misinterpreted as meaning that heat can never flow from a colder region to a hotter region, but this is not the case. The law simply states that the overall, or 'net', flow of energy will be in the direction of the temperature gradient.
The Earth is not an isolated system, as it receives a constant input of energy from the Sun. This energy is radiated back out into space, but greenhouse gases in the atmosphere, such as water vapour, carbon dioxide and methane, absorb some of this outgoing radiation and re-emit it in all directions, including back towards the Earth's surface. This means that the net flow of energy is out into space, as required by the Second Law, but the presence of greenhouse gases inhibits the cooling of the Earth's surface.
The Earth's atmosphere does not violate the Second Law of Thermodynamics. However, the law can be used to explain why the Earth's surface is warmer than it would be without an atmosphere.
Antitrust Laws: Nonprofit Sector's Friend or Foe?
You may want to see also

The Second Law of Thermodynamics and the Earth's evolution
The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. The law also states that the changes in the entropy in the universe can never be negative. The law is based on universal empirical observation concerning heat and energy interconversions.
The Earth is not an isolated system, as it is constantly receiving energy from the Sun. The Second Law of Thermodynamics does not state that the only flow of energy is from hot to cold, but that the net sum of the energy flows will be from hot to cold. This is the case for the Earth-Sun system, where the net flow of energy is outwards, from the hotter Sun to the cooler Earth and then out into the colder depths of space.
The Earth's atmosphere plays a role in this flow of energy. The atmosphere absorbs some of the Sun's radiation, particularly in the form of ultraviolet light, and reflects some of it back into space. The atmosphere also absorbs some of the infrared radiation emitted by the Earth's surface, re-emitting it in all directions, including back towards the Earth's surface. This is known as the greenhouse effect, which inhibits the cooling of the Earth's surface.
The greenhouse effect does not contradict the Second Law of Thermodynamics. The law refers to isolated systems only, and the Earth is not an isolated system. The Sun's radiation means that there is a constant energy increase on Earth, and while the Earth may be becoming more organised, the universe as a whole is becoming more disorganised as the Sun releases energy.
The Second Law of Thermodynamics also has implications for the theory of evolution. The law states that a system will become more disordered over time. This may seem to contradict the theory of evolution, which describes how organisation and complexity increase over time. However, this apparent contradiction is resolved by the fact that the Earth is not an isolated system.
Castle Law: Exemptions for Young Adults?
You may want to see also

The Second Law of Thermodynamics and the Earth's age
The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. The law also states that the changes in the entropy in the universe can never be negative. This is known as the "arrow of time", encompassing every area of science. The thermodynamic arrow of time (entropy) is the measurement of disorder within a system.
The Second Law was formulated by Rudolf Clausius in the 1850s: "Heat generally cannot flow spontaneously from a material at a lower temperature to a material at a higher temperature."
The Earth is not an isolated system, as it is constantly receiving energy from the Sun. The Earth's atmosphere is also not a closed system, as it is constantly receiving heat from the Earth's surface. Therefore, the Second Law of Thermodynamics does not contradict the existence of the Earth's atmosphere.
In the 1800s, scientists attempted to determine the age of the Earth. Lord Kelvin hypothesised that the Earth's surface was once extremely hot and was cooling slowly over time. Using the Second Law of Thermodynamics, he estimated the Earth to be at least 20 million years old. However, this estimate was inaccurate as radioactivity was not yet understood.
Internship Wage Laws: Minimum Wage Compliance
You may want to see also

The Second Law of Thermodynamics and the Earth's atmosphere
The Second Law of Thermodynamics states that the state of entropy of the entire universe, as an isolated system, will always increase over time. The law also states that the changes in the entropy in the universe can never be negative.
The Second Law of Thermodynamics applies to Earth's atmosphere. The law states that the net sum of the energy flows will be from hot to cold. In the case of the Earth-Sun system, the sunshine hits the top of the atmosphere and some of it makes it down to the surface, where it heats up the ground and the oceans. The surfaces then give off heat in the form of invisible but warming infra-red radiation. A proportion of that radiation goes back up through the atmosphere and escapes to space. However, another proportion of it is absorbed by greenhouse gas molecules, such as water vapour, carbon dioxide and methane. These molecules then re-emit that heat energy in all directions, including downwards. This process is known as the greenhouse effect, which inhibits the cooling of Earth's surface.
The Second Law of Thermodynamics can also be applied to Earth's atmosphere in the context of the age of the Earth. Lord Kelvin, a key figure in the development of the Second Law, used thermodynamics to estimate the age of the Earth. He hypothesised that the Earth's surface was once extremely hot and that it was cooling slowly over time. Using this information, he estimated the Earth to be at least 20 million years old. Although this estimate was incorrect, it was far more accurate than other estimates at the time.
Lemon Law and Private Sales: What's the Deal?
You may want to see also

The Second Law of Thermodynamics and the Earth's energy budget
The Second Law of Thermodynamics is a physical law based on empirical observation concerning heat and energy interconversions. It establishes the concept of entropy as a physical property of a thermodynamic system.
The law states that heat always flows spontaneously from hotter to colder regions of matter. It also states that not all heat can be converted into work in a cyclic process. The law predicts whether processes are forbidden despite obeying the requirement of conservation of energy as expressed in the first law of thermodynamics.
The Second Law of Thermodynamics is consistent with the greenhouse effect, which is directly observed. The greenhouse effect is a naturally occurring phenomenon where the Earth's atmosphere traps heat radiated from the Earth's surface, thereby increasing the temperature.
The Earth's energy budget is the balance of the amount of solar radiation (sunshine) that Earth receives and the amount of thermal radiation that Earth emits back into space. The greenhouse effect affects the Earth's energy budget by inhibiting the cooling of the Earth's surface, thereby increasing the temperature.
The Second Law of Thermodynamics applies to the Earth's energy budget by allowing the flow of energy from hotter to colder regions. The Earth is part of a constant, net energy flow from the Sun to the Earth and back out into space. Greenhouse gases in the Earth's atmosphere, such as carbon dioxide, water vapour, and methane, inhibit part of this net flow by returning some of the outgoing energy back towards the Earth's surface.
The Second Law of Thermodynamics does not contradict the greenhouse effect. The law states that the net sum of the energy flows will be from hot to cold, which is consistent with the greenhouse effect, where the net flow of energy is still out into space, but some energy is returned towards the Earth's surface.
How Historic Company Depictions Avoid Copyright Lawsuits
You may want to see also
Frequently asked questions
The second law of thermodynamics is a physical law based on universal empirical observation concerning heat and energy interconversions. It establishes the concept of entropy as a physical property of a thermodynamic system. It does not state that the only flow of energy is from hot to cold but that the net sum of the energy flows will be from hot to cold.
The second law of thermodynamics applies to Earth's atmosphere in the same way it applies to any other system. The Earth is not an isolated system, but part of a constant, net energy flow from the Sun, to Earth, and back out into space.
The greenhouse effect is the process by which the Earth's atmosphere inhibits the cooling of the Earth's surface by absorbing and re-emitting thermal radiation in all directions, including back towards the Earth's surface.
No, the greenhouse effect does not violate the second law of thermodynamics.







