The laws of thermodynamics are fundamental to understanding how energy is acquired, stored, and utilised by cells, and thus, all living things. The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. This is evident in cellular respiration, where energy stored in the form of glucose is released through the production of ATP, which is then used to perform essential cell functions. The second law of thermodynamics states that every energy transfer results in a loss of energy in an unusable form, such as heat, leading to a more disordered system. This is also observed in cellular respiration, where not all of the energy stored in glucose is converted to ATP, with some being lost as heat.
What You'll Learn

The First Law of Thermodynamics
During cellular respiration, cells extract energy from food, specifically from glucose, and convert it into a form that can be used to perform various cell functions. This process involves breaking down the glucose molecule and capturing the energy stored in its chemical bonds. The energy stored in glucose can be released through cellular respiration, allowing plant and animal organisms to access this energy and use it for essential tasks such as DNA replication, cell movement, and apoptosis.
In the context of cellular respiration, the breakdown of glucose is a spontaneous process. This is because it is exothermic, releasing energy, and it increases disorder by taking one highly organized molecule of glucose and converting it into multiple separate molecules. The energy released during this process is captured and used to form ATP, which is an essential energy source for cells.
Overall, the First Law of Thermodynamics emphasizes that energy is conserved in cellular respiration. While the form of energy may change, the total amount of energy remains constant. This principle is fundamental to understanding how cells acquire, store, and utilize energy to sustain life.
Copyright Laws: Monetization and Fair Use Explained
You may want to see also

The Second Law of Thermodynamics
In cellular respiration, glucose is converted into ATP (Adenosine Triphosphate). This results in a release of energy, some of which is lost as heat, in accordance with the second law of thermodynamics.
This law explains why organisms must consume large amounts of food (a source of energy), much of which ultimately dissipates as heat instead of converting directly to useful work in the form of ATP. This is why all energy transfers trend towards entropy, i.e., disorder.
The breakdown of glucose is intimately linked to the Second Law of Thermodynamics. The change in Gibbs free energy, which is defined as the enthalpy change minus the product of the temperature and entropy change, must be negative for a reaction to be favourable. This means that the change in Gibbs free energy from the initial state (glucose) to the final state (carbon dioxide, water, and energy) must be negative. The Second Law of Thermodynamics is, therefore, the reason that glucose can be broken down in a way that releases useful energy in the form of ATP.
Perpetuities Law: Trusts and Their Exemptions
You may want to see also

Energy stored in bonds
The energy used by cells is primarily the kinetic energy of electrons in the bonds of molecules. In order to make a chemical bond, energy is required to shuffle electrons around, to get them in the right configuration to form a stable bond. This energy is then said to be "stored" in the bond.
Energy storage in bonds is not entirely accurate, however. It requires energy to both make and break any bond. Where we gain energy is when the sum of energy put into breaking and forming all bonds on the reactant side of a chemical equation is less than that sum on the product side.
The energy in a chemical bond is potential energy. When such a bond is broken, and a molecule is turned back into a collection of atoms, energy is released. The energy in a chemical bond is stored energy that, when released, has the ability to do work.
The challenge for all living organisms is to obtain energy from their surroundings in forms that they can transfer or transform into usable energy to do work. Living cells have evolved to meet this challenge very well. Chemical energy stored within organic molecules such as sugars and fats transforms through a series of cellular chemical reactions into energy within ATP molecules.
ATP (adenosine triphosphate) is the most common energy "currency" in cells because the free energy change from its hydrolysis is enough to be useful to drive many otherwise endergonic reactions by coupling, but it is less costly (energetically) to make than other compounds that could potentially release even more energy.
Copyright Law: US vs UK, Who Wins?
You may want to see also

The Gibbs energy
ΔG = ΔH - TΔS
Where ΔG represents the change in Gibbs energy, ΔH is the enthalpy change (final enthalpy minus initial enthalpy), and TΔS is the product of the Kelvin temperature and the entropy change (final entropy minus initial entropy).
The spontaneity of a reaction is determined by the sign of ΔG. When ΔG is negative, a process or reaction is spontaneous, meaning it will occur without the input of external energy. In cellular respiration, the breakdown of glucose results in a negative ΔG, indicating that the reaction is spontaneous and can occur without external intervention. This spontaneity is a crucial aspect of cellular respiration, as it allows cells to access and utilise the energy stored in glucose.
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed but can only change form. In cellular respiration, this principle is evident as the energy stored in the bonds of glucose is converted into other forms, such as ATP, which cells use to perform various functions.
The second law of thermodynamics states that when energy is transferred, there will always be a loss of energy, and the overall entropy or disorder of the system will increase. In cellular respiration, the breakdown of glucose is an example of a spontaneous process that increases entropy. While the process releases energy, it is not 100% efficient, and some energy is lost as heat or reflected away.
BC Law: Application and Reach in California
You may want to see also

Entropy
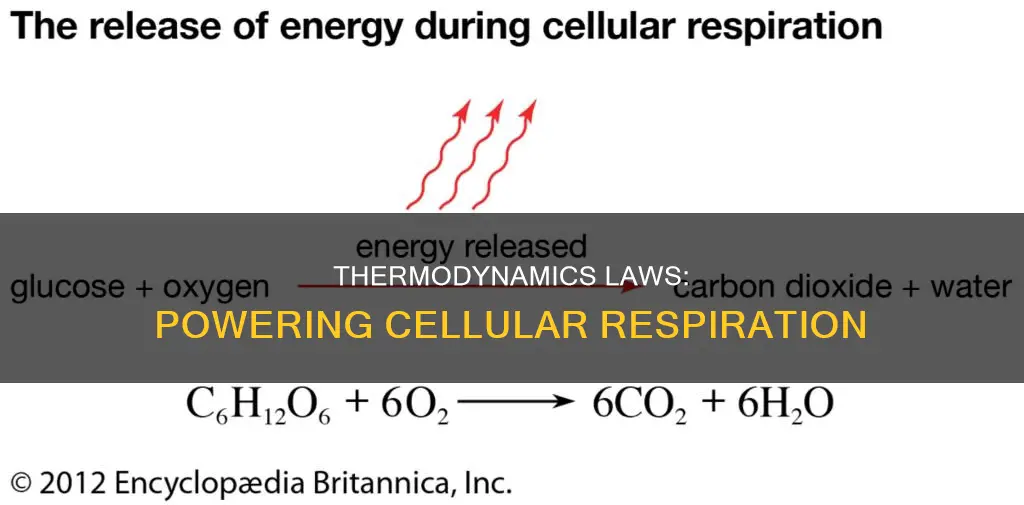
The equation for cellular respiration is:
> #C_6H_12O_6+6O_2->6CO_2+6H_2O#
There are more molecules after the reaction than before (12 compared to 7), indicating a move from relative order to disorder. The products of the reaction, H2O, also have a lower energy than the reactants, primarily glucose, making it an exothermic reaction with negative enthalpy.
The Gibbs energy, or Gibbs "free" energy, is defined in terms of enthalpy, temperature and entropy. When ΔG < 0, a process or reaction is spontaneous and will occur on its own. The change in Gibbs energy depends on the change in enthalpy (heat content) and entropy of a system.
The spontaneity of a reaction cannot be determined by its enthalpy change alone, and entropy plays a crucial role. The larger the ΔG, the more usable energy can be extracted from a reaction. Two criteria for extracting a lot of usable energy from a reaction are that it is exothermic and creates disorder from order (large entropy change).
The cellular breakdown of glucose meets these criteria, as it is exothermic and takes one highly organised molecule of glucose and six molecules of oxygen and converts them into 12 separate, simple molecules. This process results in a large and negative ΔG, meaning a large amount of usable energy can be extracted.
Ionic Bonds: Law of Multiple Proportions Applicability
You may want to see also
Frequently asked questions
The first law of thermodynamics states that the total amount of energy in the universe is constant; energy cannot be created or destroyed, but it can be transformed and transferred.
Cellular respiration is a process by which energy stored in carbohydrates, lipids, and other macromolecules is released through the production of ATP. This process allows plant and animal organisms to access the energy stored in these compounds.
The second law of thermodynamics states that every energy transfer involves some loss of energy in an unusable form, such as heat energy, resulting in a more disordered system.
During cellular metabolic reactions, some energy is lost as heat energy. This loss of energy results in an increase in disorder or entropy in the system.
Adenosine triphosphate (ATP) is an energy carrier molecule. During cellular respiration, energy stored in macromolecules is released through the production of ATP. This ATP can then be used to perform cell functions such as DNA replication, mitosis, and cell movement.







