Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases. This law is based on the kinetic theory of gases, which posits that gases in a mixture act independently and do not react with each other.
Mathematically, this law can be expressed as:
P_total = P_1 + P_2 + ... + P_n
Where P_total is the total pressure exerted by the mixture, and P_1, P_2, etc., are the partial pressures of the individual gases.
This law is particularly useful in various fields, including anaesthesia, where the partial pressures of gases in a mixture are often of interest. It also has applications in engineering, chemistry, and physics.
| Characteristics | Values |
|---|---|
| Total pressure exerted by a mixture of gases | Equal to the sum of the partial pressures of the gases in the mixture |
| Pressure of a mixture of non-reactive gases | Can be defined as the summation: p_total = p_1 + p_2 + p_3 + ... + p_n |
| Mole fraction of a specific gas in a mixture of gases | Equal to the ratio of the partial pressure of that gas to the total pressure exerted by the gaseous mixture |
| Mole fraction | Can be used to calculate the total number of moles of a constituent gas when the total number of moles in the mixture is known |
| Mole fraction | Can be used to calculate the volume occupied by a specific gas in a mixture |
What You'll Learn
- The total pressure of a gas mixture is the sum of the partial pressures of constituent gases
- Dalton's Law is related to the ideal gas laws
- The pressure of a non-reactive gas mixture can be defined as the summation of its component pressures
- Dalton's Law is not strictly followed by real gases
- The law can be used to determine the volume-based concentration of an individual gaseous component

The total pressure of a gas mixture is the sum of the partial pressures of constituent gases
Dalton's Law of Partial Pressures, also known as Dalton's Law, is a gas law that can be applied to a mixture of gases. It states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture.
Mathematically, this can be expressed as:
Ptotal = P1 + P2 + P3 + ... + Pn
Where:
- Ptotal is the total pressure exerted by the mixture of gases
- P1, P2, P3, etc., are the partial pressures of the individual gases in the mixture
For example, if we have a mixture of two gases, A and B, the total pressure exerted by the mixture is equal to the sum of the individual partial pressures exerted by gas A and gas B.
This law is based on the kinetic theory of gases, which states that a gas will diffuse to fill up the space it is in and that there are no forces of attraction between the molecules. Therefore, the molecules in a mixture of gases act independently and do not react with each other.
The pressure exerted by a gas is determined by its collisions with the container walls and not by collisions with molecules of other substances. Thus, each gas in a mixture exerts its own pressure, which can be added up to find the total pressure of the mixture.
Dalton's Law can be applied to calculate the total pressure of a gas mixture when the partial pressures of the constituent gases are known. It can also be used to find the partial pressure of a specific gas in a mixture when the total pressure is known.
Additionally, Dalton's Law can be applied to the total number of moles in a gas mixture if the other values, such as temperature and volume, are constant. In this case, the total number of moles is equal to the sum of the number of moles of the individual gases.
It is important to note that Dalton's Law assumes ideal gas behaviour, where gases have very low pressure and high temperature. At high pressures and low temperatures, gases may deviate from ideal behaviour, and Dalton's Law may not be applicable.
City Laws: Do They Apply in Unincorporated Counties?
You may want to see also

Dalton's Law is related to the ideal gas laws
Dalton's Law, also known as the Law of Partial Pressures, is closely related to the ideal gas laws. It states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the gases in the mixture.
Mathematically, this can be expressed as:
\\[P_{total} = P_A + P_B + ... \]
Where:
- Ptotal is the total pressure exerted by the mixture of gases
- PA, PB, etc., are the partial pressures of the individual gases in the mixture
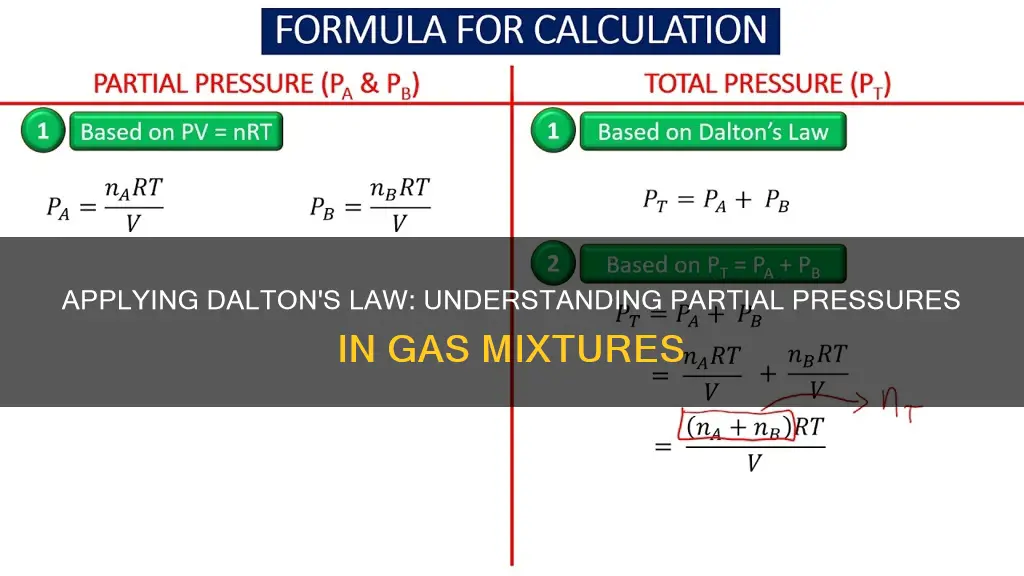
This relationship is derived from the ideal gas law:
\\[PV = nRT\]
Where:
- P is pressure
- V is volume
- N is the number of moles
- R is the gas constant
- T is temperature
If we know the molar composition of the gas mixture, we can express the total number of moles as the sum of the number of moles of each individual gas:
\\[n_{total} = n_A + n_B + ...\]
This is based on the kinetic theory of gases, which states that gases will expand to fill the container they are in without interacting with each other. Therefore, the total volume of the gas mixture can be found by multiplying the total number of moles by the gas constant and temperature and dividing by the total pressure:
\\[P_{total} V = n_{total} RT\]
This equation can be rearranged to find the total number of moles in a gas mixture.
Dalton's Law is also used to determine the mole ratio, or Xi, of a specific gas in a mixture. This is calculated as:
\\[X_i = \frac{P_i}{P_{total}} = \frac{n_i}{n_{total}} = \frac{V_i}{V_{total}}\]
Where Xi is the mole fraction of gas i in the mixture. The mole ratio represents the fraction of the mixture made up by a specific gas and can be used to determine the composition of gases in a mixture.
In summary, Dalton's Law of Partial Pressures is closely related to the ideal gas laws. It allows us to calculate the total pressure of a gas mixture by summing the partial pressures of the individual gases, and it provides a way to determine the mole ratio of a specific gas in the mixture. These calculations are based on the ideal gas law and the kinetic theory of gases.
Gas Laws: Understanding Scuba Diving Safety
You may want to see also

The pressure of a non-reactive gas mixture can be defined as the summation of its component pressures
Dalton's Law of Partial Pressures, discovered by John Dalton in 1801, states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture. This empirical law is related to the ideal gas laws.
Mathematically, the pressure of a mixture of non-reactive gases can be defined as the summation of its component pressures:
\[P_{total} = \sum_{i=1}^{n}p_{i} = p_1 + p_2 + p_3 + \dots + p_n\]
Where p1, p2, ..., pn represent the partial pressures of each component.
The partial pressure of a gas is a measure of the thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone.
The pressure of an ideal gas is determined by its collisions with the walls of its container, and not by collisions with the molecules of other substances. This is because the molecules in a mixture of gases are so far apart that they act independently and do not react with each other.
Dalton's Law can be applied to the number of moles so that the total number of moles equals the sum of the number of moles of the individual gases. Here, the pressure, temperature, and volume are held constant in the system.
The mole fraction of a specific gas in a mixture of gases is equal to the ratio of the partial pressure of that gas to the total pressure exerted by the gaseous mixture. This mole fraction can also be used to calculate the total number of moles of a constituent gas when the total number of moles in the mixture is known.
Lemon Law and Audio Equipment: What's Covered?
You may want to see also

Dalton's Law is not strictly followed by real gases
Dalton's Law of Partial Pressures states that the total pressure exerted by a mixture of You may want to see also Dalton's Law of Partial Pressures states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. This empirical law was observed by John Dalton in 1801 and published in 1802. Mathematically, the pressure of a mixture of non-reactive gases can be defined as: \\[p_{\text{total}} = p_1 + p_2 + p_3 + ... + p_n\] Where p1, p2, ..., pn represent the partial pressures of each component. \\[p_i = p_{\text{total}}c_i\] Where ci is the concentration of component i. It is important to note that Dalton's law is not strictly followed by real gases, with deviations becoming more significant at higher pressures. This is because the volume occupied by the molecules becomes substantial compared to the free space between them, increasing intermolecular forces and substantially changing the pressure exerted. You may want to see also Dalton's Law of Partial Pressures is a gas law that states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures exerted by each individual gas in the mixture. The law can be expressed as: Ptotal = P1 + P2 + P3 + ... + Pn Where Ptotal is the total pressure exerted by the mixture, and P1, P2, etc. are the partial pressures of the individual gases. The mole fraction of a specific gas in a mixture is equal to the ratio of its partial pressure to the total pressure exerted by the mixture. This can be expressed as: Xi = Pi / Ptotal = Vi / Vtotal = ni / ntotal Where Xi is the mole fraction, n is the number of moles, P is pressure, and V is volume. Let's say we have a mixture of hydrogen and oxygen gas, with a total pressure of 1.5 atm, and the partial pressure of hydrogen is 1 atm. We can use Dalton's Law to find the mole fraction of oxygen: Ptotal = Phydrogen + Poxygen Poxygen = 1.5 atm - 1 atm = 0.5 atm Xoxygen = Poxygen / Ptotal = 0.5/1.5 = 0.33 So, the mole fraction of oxygen in the mixture is 0.33.Exploring Legal Boundaries in the Ocean

The law can be used to determine the volume-based concentration of an individual gaseous component
Understanding Ohm's Law in Series Circuits
Frequently asked questions