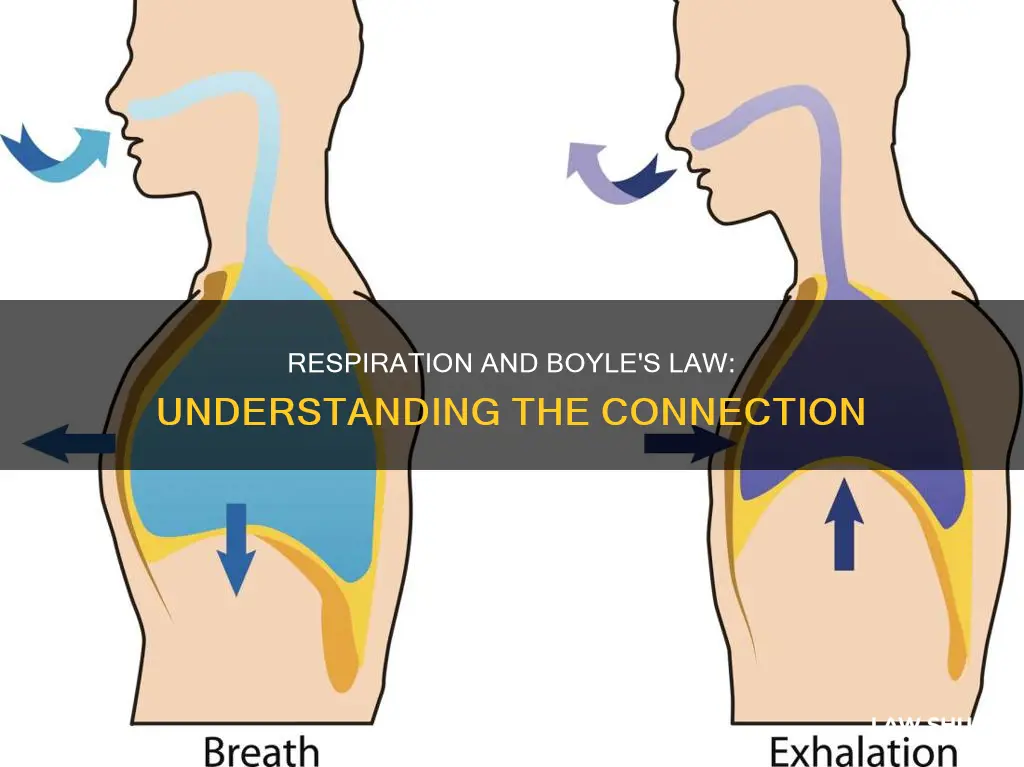
Boyle's law states that the volume of a gas and pressure are inversely proportional at a given temperature. This means that when the pressure increases, the volume decreases, and vice versa. This law is crucial to understanding the relationship between pressure and volume in the lungs during respiration. When we inhale, our lung volume increases, resulting in lower pressure, which allows air to rush in and fill the lungs. Conversely, during exhalation, the volume inside the lungs decreases, leading to an increase in pressure, causing air to move out. The inverse relationship between pressure and volume, as described by Boyle's law, is what enables the human respiratory system to function effectively.
What You'll Learn
- How does the volume of the lungs change during inspiration?
- How does the pressure inside the lungs change during exhalation?
- How does Boyle's Law explain the movement of air during inhalation?
- How does the intrapleural pressure change during inspiration?
- How does the compliance of the lung tissue change at low and high volumes?

How does the volume of the lungs change during inspiration?
Boyle's law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law is important in understanding respiration, as it explains the relationship between pressure and volume in the lungs when breathing.
During inspiration, or inhalation, the volume of the lungs increases, which results in lower pressure. This is due to the contraction of the diaphragm and the expansion of the chest cavity. The diaphragm is a large, flat muscle at the base of the rib cage that separates the thoracic cavity from the abdominal cavity. When it contracts, it pulls downward, expanding the volume of the thoracic cavity and decreasing the pressure in the lungs. This creates a vacuum, which pulls air into the lungs through the respiratory tract.
As the volume of the lungs increases, the pressure inside decreases, following Boyle's law. This decrease in pressure within the lungs causes air at atmospheric pressure to rush in and fill the lungs. The air enters the lungs through the nasal cavities or mouth, passing through the pharynx, larynx, trachea, bronchi, and bronchioles, until it reaches the alveoli, where gas exchange occurs.
The increase in lung volume during inspiration is largely due to the increase in alveolar space. The bronchioles and bronchi are stiff structures that do not change much in size. The alveoli, on the other hand, are elastic and can expand to accommodate the incoming air. This expansion of the alveoli is what primarily contributes to the increase in lung volume during inhalation.
In summary, during inspiration, the contraction of the diaphragm and expansion of the chest cavity lead to an increase in the volume of the thoracic cavity and a decrease in pressure within the lungs. This change in volume and pressure follows Boyle's law and creates a vacuum, causing air to rush into the lungs through the respiratory tract. The increase in lung volume is mainly due to the expansion of the alveoli, which are elastic and can accommodate more air.
Anti-Discrimination Law: Does It Protect White People?
You may want to see also

How does the pressure inside the lungs change during exhalation?
Boyle's law states that the volume of a gas and the pressure exerted on it are inversely proportional at a given temperature. In other words, when the pressure exerted on a gas increases, its volume decreases, and vice versa. This law is important for understanding the relationship between pressure and volume in the lungs during breathing.
During inhalation, the volume of the lungs increases, which results in lower pressure inside the lungs. This lower pressure allows air at atmospheric pressure to rush into the lungs and fill them. The diaphragm contracts and pulls downward toward the abdominal cavity, expanding the volume of the thoracic cavity. This decrease in pressure creates a vacuum, pulling air into the lungs.
During exhalation, the opposite process occurs. The volume inside the lungs decreases, and the pressure inside increases. As a result, the air moves out of the lungs. The diaphragm relaxes and moves upward into the thoracic cavity, reducing the volume of the thoracic cavity and increasing the pressure within it. This increase in pressure forces the air out of the lungs and back into the atmosphere. The movement of air out of the lungs is a passive process, with no muscles contracting to expel the air.
The lungs are elastic, and when they fill with air, the elastic recoil within the lung tissues exerts pressure back toward the interior of the lungs. Upon exhalation, the lungs recoil to force the air out, and the diaphragm and intercostal muscles relax, allowing the air to exit the body through the nasal passages or mouth.
Thus, according to Boyle's law, the pressure inside the lungs increases during exhalation as the volume decreases, and this increase in pressure forces the air out of the lungs.
Understanding EEO Laws: Do They Apply to Management?
You may want to see also

How does Boyle's Law explain the movement of air during inhalation?
Boyle's Law states that the volume of a gas and pressure are inversely proportional at a given temperature. In other words, when the pressure increases, the volume decreases, and vice versa. This law is important because it helps us understand the relationship between pressure and volume in the lungs when breathing.
During inhalation, the diaphragm contracts and pulls downward toward the abdominal cavity, expanding the volume of the thoracic cavity. This decreases the pressure in the lungs and creates a vacuum, which pulls air into the lungs. This air can enter the respiratory tract through the nasal cavities or mouth, travelling into the pharynx, larynx, trachea, bronchi, and bronchioles, and finally into the alveoli, where gas exchange occurs.
According to Boyle's Law, as the volume of the thoracic cavity increases due to the contraction of the diaphragm and expansion of the chest wall, the pressure must decrease. Therefore, as the intrapleural volume increases, the intrapleural pressure decreases, allowing air to flow into the lungs for gas exchange.
The human body brings air into the lungs by creating negative pressure. During inhalation, the contraction of the diaphragm and external intercostal muscles increases the volume of the thorax. As the volume increases, the pressure decreases, and air rushes in to fill the lungs. This movement of air during inhalation is explained by Boyle's Law, which describes the inverse relationship between volume and pressure.
Copyright Law: Non-Profits and Legal Protection
You may want to see also

How does the intrapleural pressure change during inspiration?
During inspiration, the diaphragm and external intercostal muscles contract, increasing the volume of the thorax. This contraction causes the lungs to expand as the thorax expands. According to Boyle's law, as the volume of the lungs increases, the pressure within the lungs decreases, allowing air at atmospheric pressure to rush into the lungs. Therefore, during inspiration, the intrapleural pressure decreases.
At rest, the intrapleural pressure is near -5 cm H2O. During inspiration, the contraction of the diaphragm and external intercostal muscles causes this pressure to decrease to about -8 cm H2O at the end of inspiration. This decrease in intrapleural pressure is what allows air to enter the lungs for gas exchange.
The intrapleural pressure is the pressure within the intrapleural space, which is the space between the parietal pleura and visceral pleura surrounding the lungs. This pressure is usually slightly less than the atmospheric pressure, which is known as negative pressure. This negative pressure is essential for keeping the lungs expanded, even during exhalation. If humans did not maintain this slightly negative pressure, their lungs would collapse as air rushed towards the area of lower pressure.
The change in intrapleural pressure during inspiration can be understood through Boyle's law, which states that the volume of a gas and its pressure are inversely proportional at a given temperature. In other words, as the volume of a gas increases, its pressure decreases, and vice versa. This principle is crucial in understanding how the human respiratory system functions.
The Universal Gas Law: Does It Exist?
You may want to see also

How does the compliance of the lung tissue change at low and high volumes?
Lung compliance refers to the ability of the lungs to stretch and expand in response to a change in intrapleural pressure. It is a measure of the distensibility of the lungs, chest wall, and abdominal viscera. Compliance is highest at moderate lung volumes and is much lower at very low or very high volumes.
The compliance of the lung tissue is dependent on two factors: the elasticity of the elastin in the connective tissue and the surface tension of the alveoli, which is decreased by surfactant production.
At low volumes, the lung is less compliant or distensible. This is due to the effect of surface tension, which increases the pressure required to inflate the alveoli. As the alveolar radius is smaller at low volumes, a greater inward force is generated, which must be opposed by alveolar pressure to prevent alveolar collapse.
At high volumes, the lung also becomes less compliant. This is due to the elastic limit of the lung tissue being reached, similar to an overstretched elastic band becoming harder to stretch further.
Thus, the lung is most compliant at moderate volumes, where the slope of the compliance curve is steepest, indicating that a relatively small change in pressure will lead to a large change in volume. This is the normal operating range for breathing, as it requires less work from the respiratory muscles to inflate the lungs.
Phone Laws: Exempting Tesla Drivers?
You may want to see also
Frequently asked questions
Boyle's law states that the volume of a gas and pressure are inversely proportional to each other when the temperature and the amount of gas are kept constant.
During inhalation, the diaphragm contracts, and the volume of the thoracic cavity increases, resulting in lower pressure. This allows air to rush into the lungs. During exhalation, the diaphragm relaxes, the volume of the thoracic cavity decreases, and the pressure increases, forcing air out of the lungs.
The mathematical representation of Boyle's law is: $P\propto \dfrac{1}{V}$, where P is pressure and V is volume.
The human respiratory system functions by bringing air into the lungs through negative pressure. The contraction of inspiratory muscles, such as the diaphragm and external intercostal muscles, increases the volume of the thorax, resulting in a decrease in pressure, which allows air to flow into the lungs.
As a scuba diver descends into the water, the pressure on their lungs increases, and according to Boyle's law, the air volume inside the lungs must decrease. As the diver ascends, the pressure decreases, and the air volume inside the lungs increases. It is important for the diver to exhale steadily during this process to release the volume of gas and prevent pulmonary barotrauma.