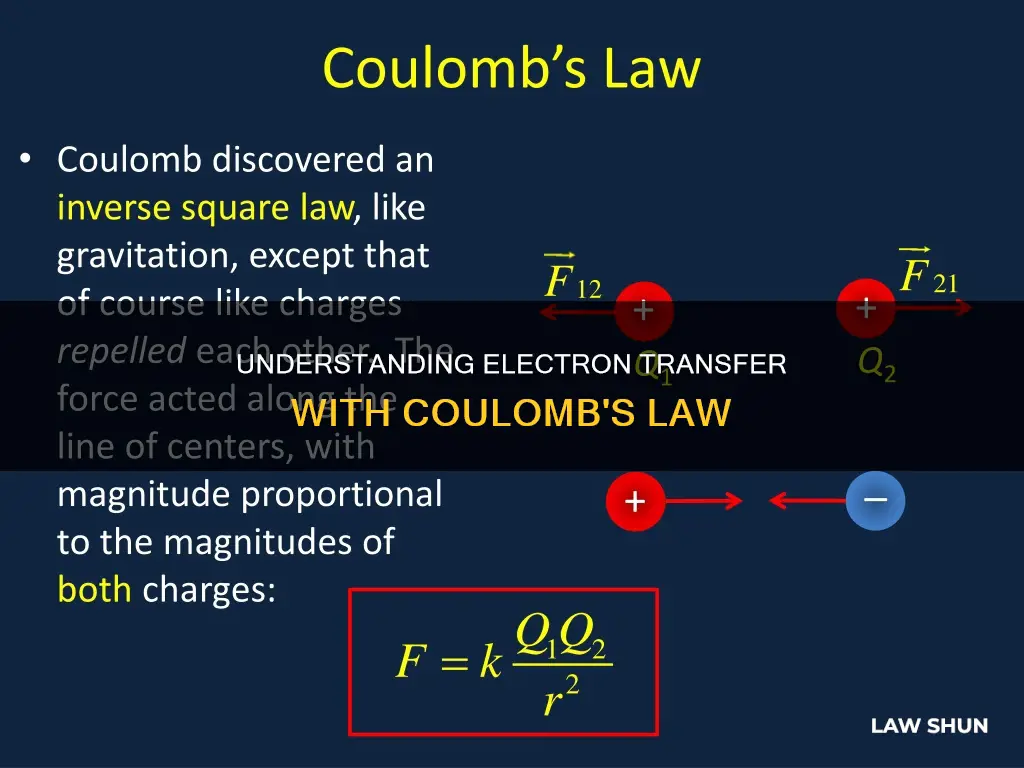
Coulomb's Law is a fundamental concept in physics and chemistry, describing the electric force between charged objects. It was formulated by French physicist Charles-Augustin de Coulomb in the 18th century and is analogous to Newton's law of gravity. The law states that the force between two electrically charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them. This means that as the distance between charges increases, the force of attraction decreases, and vice versa.
Coulomb's Law is essential in understanding why atoms transfer electrons. In an atom, the negatively charged electrons are attracted to the positively charged protons in the nucleus, as predicted by Coulomb's Law. This transfer of electrons is influenced by the magnitude of the charges and the distance between them.
| Characteristics | Values |
|---|---|
| What is Coulomb's Law? | An experimental law of physics that calculates the amount of force between two electrically charged particles at rest. |
| Who was it named after? | French physicist Charles-Augustin de Coulomb |
| What does it state? | The magnitude, or absolute value, of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. |
| What is the formula for Coulomb's Law? | F=k * (q1 * q2) / r^2 |
| What do the variables in the formula represent? | F = force, k = Coulomb's constant, q1 and q2 = the magnitudes of the charges, r = the distance between the charges |
| What is the value of Coulomb's constant? | 8.987 * 109 Nm2/C^2 |
| What are the implications of Coulomb's Law? | Opposite charges attract, like charges repel; the force is proportional to the magnitude of both charges; the force is inversely proportional to the square of the distance between the two charges; if the two charges have the same sign, the force is repulsive, if they have different signs, the force is attractive |
| How does Coulomb's Law apply to atoms? | It describes the force between the positively charged atomic nucleus and each of the negatively charged electrons. |
What You'll Learn
- Coulomb's Law describes the force between two electrically charged particles at rest
- The magnitude of the force is directly proportional to the magnitude of both charges
- The force is inversely proportional to the square of the distance between the two charges
- Like charges repel each other; unlike charges attract
- Coulomb's Law can be used to calculate ionization energy

Coulomb's Law describes the force between two electrically charged particles at rest
Coulomb's Law, or Coulomb's inverse-square law, is a fundamental law of physics that describes the force between two electrically charged particles at rest. It was first published in 1785 by French physicist Charles-Augustin de Coulomb, and it holds even within atoms.
The law states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. This can be expressed mathematically as:
|F| = ke * (|q1| * |q2|) / r^2
Where:
- F is the force
- Ke is a constant (Coulomb's constant)
- Q1 and q2 are the quantities of each charge
- R is the distance between the charges
The force acts along the straight line joining the two charges. If the charges have the same sign, they repel each other; if they have different signs, they attract each other.
Coulomb's Law is analogous to Isaac Newton's law of gravity, but there are some key differences. Both gravitational and electric forces decrease with the square of the distance between the objects, and both forces act along a line between them. However, in Coulomb's Law, the magnitude and sign of the electric force are determined by the electric charge of an object, rather than its mass.
Coulomb's Law is essential to understanding why atoms transfer electrons. In an atom, the positively charged atomic nucleus attracts the negatively charged electrons towards it. The force of this attraction is described by Coulomb's Law. The closer an electron is to the nucleus, the stronger the attractive force, and the more negative the force (F) becomes.
Additionally, the magnitude of the force is directly related to the magnitude of the charges on the particles. So, the more protons in the nucleus, the stronger the force of attraction. This is why atoms with larger nuclei tend to have higher ionization energies—because it requires more energy to overcome the stronger Coulombic attraction.
Coulomb's Law also helps explain why electrons in outer shells have lower ionization energies. Due to the inverse-square relationship in the law, the force of attraction decreases as the distance between the charges increases. So, electrons in outer shells, which are farther from the nucleus, experience a weaker attractive force and therefore have lower ionization energies.
Labor Laws: Global Business Compliance Challenges
You may want to see also

The magnitude of the force is directly proportional to the magnitude of both charges
Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. The law states that the magnitude of the force is directly proportional to the magnitude of both charges and inversely proportional to the square of the distance between them. In other words, the greater the charge, the stronger the force, and the larger the distance, the weaker the force.
The law can be expressed mathematically as:
F=k * (q1 * q2) / r^2
Where:
- F is the force
- K is Coulomb's constant
- Q1 and q2 are the magnitudes of the charges
- R is the distance between the charges
Coulomb's Law is important in chemistry as it helps explain why electrons don't leave atoms. Electrons are attracted to the protons in the nucleus, as predicted by Coulomb's Law. The specifics of the law, such as the inverse-square law and the magnitude of the charges, become relevant when calculating ionization energy, or the energy needed to remove an electron from an atom.
Employment Discrimination Law: Resident Aliens' Rights Explored
You may want to see also

The force is inversely proportional to the square of the distance between the two charges
Coulomb's Law, or Coulomb's inverse-square law, is a fundamental principle in physics that describes the force between two electrically charged particles at rest. The law was first published in 1785 by French physicist Charles-Augustin de Coulomb, and it holds even within atoms.
The law states that the magnitude of the attractive or repulsive force between two charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. This relationship is described by the equation:
\[ F=k \dfrac{q_1q_2}{r^2} \]
Where:
- \(F\) is the force
- \(k\) is Coulomb's constant
- \(q_1\) and \(q_2\) are the magnitudes of the charges
- \(r\) is the distance between the charges
The inverse-square relationship means that if the distance between the two charges is doubled, the force becomes four times weaker. Conversely, if the charges are brought ten times closer together, the force becomes 100 times stronger.
In the context of atoms, Coulomb's Law explains the force between the positively charged atomic nucleus and the negatively charged electrons. The attractive force between the nucleus and an electron is much stronger than the repulsive force between electrons. This is because the distance between the nucleus and an electron is much smaller than the distance between electrons.
Mendel's Law: Sexual vs Asexual Reproduction
You may want to see also

Like charges repel each other; unlike charges attract
Coulomb's Law, an experimental law of physics, calculates the amount of force between two electrically charged particles at rest. The law was first published in 1785 by French physicist Charles-Augustin de Coulomb.
Coulomb's Law states that the magnitude of the attractive or repulsive electrostatic force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them.
In the context of subatomic particles, this means that protons repel protons, electrons repel electrons, and protons attract electrons (and vice versa). This is because electrons are negatively charged, while atomic nuclei are positively charged.
The force exerted by one particle on another is equal in both directions (Newton's Third Law). The force is proportional to the magnitude of both charges. If the two charges have the same sign (positive or negative), the force is repulsive; if they have different signs, the force is attractive.
The size of the force varies inversely as the square of the distance between the two charges. Therefore, if the distance between the two charges is doubled, the attraction or repulsion becomes weaker, decreasing to one-fourth of the original value. If the charges come ten times closer, the size of the force increases by a factor of 100.
The Amish and the Law: A Complex Relationship
You may want to see also

Coulomb's Law can be used to calculate ionization energy
Coulomb's Law is an experimental law of physics that calculates the amount of force between two electrically charged particles at rest. The law states that the magnitude of the attractive or repulsive force between two point charges is directly proportional to the product of the magnitudes of their charges and inversely proportional to the square of the distance between them. The scalar equation for Coulomb's Law is:
F=k * (q1 * q2) / r^2
Where:
- F is the force
- K is Coulomb's constant
- Q1 and q2 are the magnitudes of the charges
- R is the distance between the particles
Coulomb's Law is important in chemistry because it helps explain why electrons remain in atoms. Electrons are attracted to the positively charged nucleus of an atom, and removing an electron requires energy to overcome this attraction, known as the ionization energy.
Coulomb's Law can be used to calculate the ionization energy of an atom. Ionization energy is the energy needed to remove an electron from an atom, creating an ion. The protons in the nucleus attract the electrons, so taking away an electron requires energy to overcome that attraction. The magnitude of the ionization energy depends on the number of protons in the nucleus and the distance from the electron to the nucleus.
For example, let's consider an atom composed of a proton and an electron separated by 185.00 pm. The ionization energy can be calculated using the following values:
- K = 9 × 10^9 Nm^2C-2
- Q1 = charge on the proton = 1.6 × 10^-19 C
- Q2 = charge on the electron = -1.6 × 10^-19 C
- R = distance of separation = 185 × 10^-12 m
By substituting these values into the equation, we can calculate the ionization energy.
Coulomb's Law also helps explain why electrons occupy different shells in an atom. Electrons in outer shells are farther from the nucleus, so Coulomb's Law predicts that they will have a lower ionization energy compared to electrons in inner shells.
Homeschooling Laws and the Amish: Do They Apply?
You may want to see also
Frequently asked questions
Coulomb's Law is a mathematical description of the electric force between charged objects. It states that the magnitude of the attractive or repulsive force between two charged particles is directly proportional to the product of their charges and inversely proportional to the square of the distance between them.
Opposite charges attract, and like charges repel. This means that in the context of subatomic particles, protons attract electrons, and protons repel protons, and electrons repel electrons.
In an atom, the positively charged protons in the nucleus attract the negatively charged electrons. The force of this attraction depends on the distance between the particles and the magnitude of their charges.
Atoms can transfer electrons due to the attractive and repulsive forces described by Coulomb's Law. If an electron is far enough away from the nucleus, it can be attracted to a different nucleus with a stronger positive charge.
Ionization energy is the energy needed to remove an electron from an atom. Coulomb's Law predicts that this will require energy to overcome the attractive force between the positively charged nucleus and the negatively charged electron.







