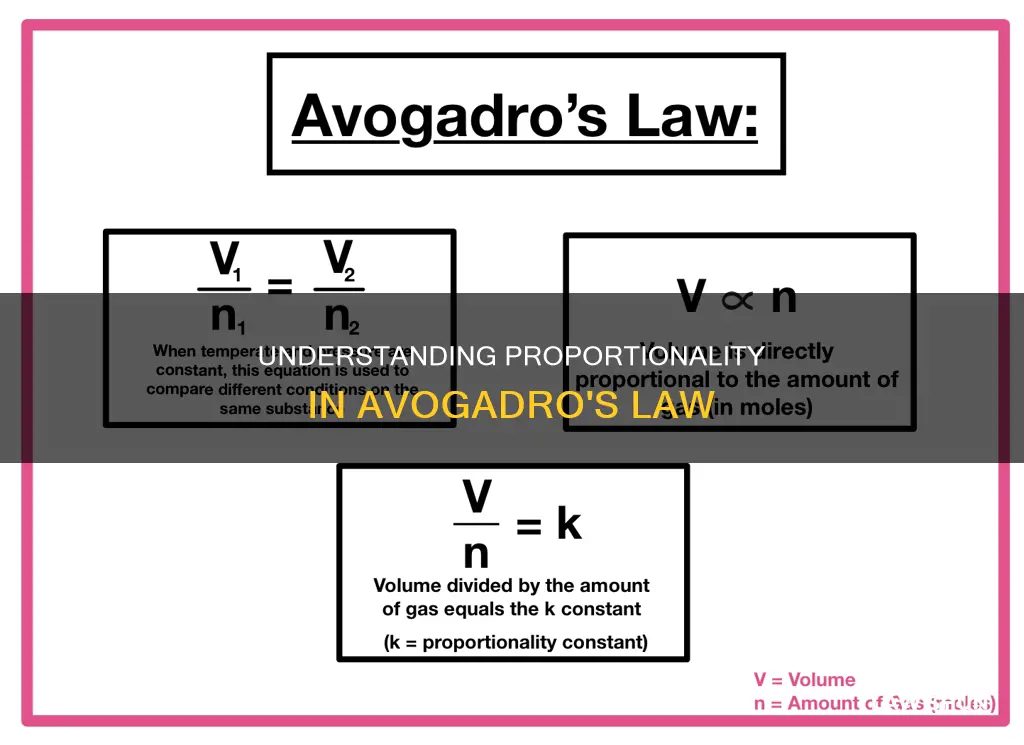
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance in it. It states that equal volumes of all gases at the same temperature and pressure have the same number of molecules. In other words, the volume of a gas is directly proportional to the amount of the gas in moles. This law is a specific case of the ideal gas law and can be expressed mathematically as V ∝ n, where V is the volume of the gas and n is the amount of substance of the gas (measured in moles). This law is named after Amedeo Avogadro, who hypothesised it in 1811 or 1812, and it has been instrumental in establishing the formulas of simple molecules and determining atomic and molecular masses.
| Characteristics | Values |
|---|---|
| Volume of gas | Directly proportional to the amount of gas (moles) at constant temperature and pressure |
| Volume of gas | Inversely proportional to pressure at constant temperature and number of moles |
| Volume of gas | Directly proportional to temperature at constant pressure and number of moles |
What You'll Learn

Volume and amount of gas are directly proportional
Avogadro's Law, also known as Avogadro's hypothesis or principle, is a gas law that relates the volume of a gas to the amount of substance in the gas. It is a specific case of the ideal gas law.
The law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." This means that for a given mass of an ideal gas, the volume and amount of the gas (in moles) are directly proportional if the temperature and pressure are constant. This can be expressed mathematically as:
> V ∝ n
Where V is the volume of the gas and n is the amount of substance of the gas (measured in moles).
Avogadro's Law can also be written as:
> V/n = k
Here, k is a constant for a given temperature and pressure. This equation shows that as the number of moles of gas increases, the volume of the gas also increases proportionately. Similarly, if the number of moles of gas is decreased, the volume decreases as well.
For example, consider a balloon filled with 0.0920 mol of helium gas to a volume of 1.90 L. If we add 0.0210 mol of additional helium while keeping the temperature and pressure constant, the new volume of the balloon will be 2.33 L. This demonstrates the direct proportionality between the volume and amount of gas according to Avogadro's Law.
Avogadro's Law is a crucial concept in chemistry, especially in understanding the behaviour of gases and the formulas of simple molecules. It provides a way to calculate the quantity of gas in a container and has practical applications, such as determining the correct amount of air to put into a tire to maintain its shape and avoid bursting.
The Royal Law: James' Admonition Explained
You may want to see also

Volume and temperature are directly proportional
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance in it. It is a specific case of the ideal gas law. The law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." This means that for a given mass of an ideal gas, the volume and amount of the gas (in moles) are directly proportional when the temperature and pressure are constant.
The law can be expressed mathematically as:
- V ∝ n
- V/n = k
- V1/n1 = V2/n2
Where V is the volume of the gas, n is the amount of substance (in moles), and k is a constant for a given temperature and pressure.
Avogadro's Law can be combined with other gas laws, such as Charles's Law and Boyle's Law, to form the ideal gas law. Charles's Law states that at constant pressure, temperature and volume are directly proportional. Boyle's Law describes the inverse relationship between the pressure and volume of a fixed amount of gas at a constant temperature.
According to Avogadro's Law, the volume of a gas is directly proportional to the number of moles of gas present, when temperature and pressure are held constant. This means that as the number of moles of gas increases, the volume of the gas also increases proportionally. Similarly, if the number of moles of gas decreases, the volume decreases as well. This relationship between volume and temperature can be observed when blowing up a balloon. As more gas (in moles) is added to the balloon, the volume of the balloon increases.
Therefore, Avogadro's Law demonstrates that volume and temperature are directly proportional when dealing with gases, as long as pressure and the amount of substance remain constant.
Applicable Laws in Arbitration: A Comprehensive Guide
You may want to see also

Volume and pressure are inversely proportional
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance in it. The law is a specific case of the ideal gas law.
Avogadro's Law states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." This means that for a given mass of an ideal gas, the volume and amount of the gas (in moles) are directly proportional if the temperature and pressure remain constant.
The law can be written as:
V ∝ n
V/n = k
Where V is the volume of the gas, n is the amount of substance (in moles), and k is a constant for a given temperature and pressure.
This law describes how, under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules.
Avogadro's Law can be derived from the ideal gas law:
PV = nRT
Where R is the gas constant, T is the temperature in Kelvin, and P is the pressure in pascals. Solving for V/n gives:
V/n = RT/P
So, k = RT/P, which is a constant for a fixed pressure and temperature.
Now, to address the relationship between volume and pressure in the context of Avogadro's Law:
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the pressure and volume after a change.
This inverse relationship between volume and pressure, as described by Boyle's Law, is an important aspect of understanding gas behaviour. It shows that when the volume of a gas decreases, its pressure increases, and vice versa. This relationship holds true as long as the number of molecules and the temperature remain constant.
In summary, while Avogadro's Law focuses on the direct proportionality between volume and the amount of substance (in moles) at constant temperature and pressure, Boyle's Law complements this by describing the inverse relationship between volume and pressure when the amount of substance and temperature are held constant. Together, these laws provide valuable insights into the behaviour of gases and how changes in volume, pressure, temperature, and the number of molecules influence each other.
Who Does the Anti-Kickback Law Apply To?
You may want to see also

Avogadro's Law and Charles' Law are compatible
Avogadro's Law and Charles's Law are indeed compatible, and they are both special cases of the ideal gas law.
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance of gas present. It is named after Amedeo Avogadro, who, in 1811 or 1812, hypothesised that two samples of an ideal gas with the same volume, temperature, and pressure contain the same number of molecules. The law can be written as:
${\displaystyle V\propto n}$
${\displaystyle {\frac {V}{n}}=k}$
Where V is the volume of the gas, n is the amount of substance of the gas (measured in moles), and k is a constant for a given temperature and pressure.
Charles's Law, on the other hand, states the relationship between the volume and temperature of a gas. It was first discovered by the French chemist Jacques Alexandre César Charles in 1783, and later studied by Joseph-Louis Gay-Lussac in 1808. Charles's Law can be written as:
${\displaystyle V ={\rm const.}\; T}$
${\displaystyle V \propto T}$
Where V is the volume of the gas, T is the temperature in Kelvin, and the two are directly proportional to each other at constant pressure.
Both Avogadro's Law and Charles's Law describe a proportionality of the volume of a gas when the pressure is constant. The volume of a gas is directly proportional to the amount of substance of the gas (Avogadro's Law) and to the temperature (Charles's Law). This means that Avogadro's Law and Charles's Law are compatible and can be combined to form the ideal gas law, along with Boyle's Law.
Conflict Management Laws: Understanding UK's Legal Framework
You may want to see also

Avogadro's Law and Boyle's Law are compatible
Avogadro's Law and Boyle's Law are indeed compatible, and they are both special cases of the ideal gas law.
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance of gas present. It states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules." In other words, for a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
Boyle's Law, on the other hand, describes the relationship between the pressure and volume of a gas. It states that at a constant temperature, the volume of a fixed amount of gas is inversely proportional to its pressure. In mathematical terms, this can be expressed as:
> PV = constant
> V ∝ 1/P
> V = constant/P
Both Avogadro's Law and Boyle's Law deal with the behaviour of gases, but they focus on different variables. Avogadro's Law relates the volume of a gas to the amount of substance, while Boyle's Law relates the volume to the pressure. Despite their different focuses, these laws are compatible because they are both special cases of the ideal gas law, which takes into account the pressure, volume, temperature, and amount of a gas.
The ideal gas law combines several gas laws, including Avogadro's Law and Boyle's Law, into a single equation:
> PV = nRT
Here, P is the pressure, V is the volume, n is the number of gas molecules in moles, T is the temperature in Kelvin, and R is the ideal gas constant. This equation demonstrates how all these variables are interrelated and how changes in one variable can affect the others.
In summary, Avogadro's Law and Boyle's Law are compatible because they are both components of the broader ideal gas law, which describes the complex behaviour of gases in a comprehensive manner.
HIPAA Laws: Do They Apply to Counselors?
You may want to see also
Frequently asked questions
Avogadro's Law states that the volume of a gas is directly proportional to the number of moles of the gas, assuming the temperature and pressure remain constant. This can be expressed mathematically as V ∝ n, where V is the volume and n is the number of moles.
Avogadro's Law, also known as Avogadro's hypothesis or principle, is an experimental gas law that relates the volume of a gas to the amount of substance in the gas. It was formulated by Amedeo Avogadro in 1811/1812 and states that "equal volumes of all gases, at the same temperature and pressure, have the same number of molecules."
Avogadro's Law is a specific case of the Ideal Gas Law, which combines Charles's Law, Avogadro's Law, and Boyle's Law. While Avogadro's Law relates volume to the number of moles, Boyle's Law describes the inverse relationship between volume and pressure, and Charles's Law describes the direct relationship between volume and temperature.







