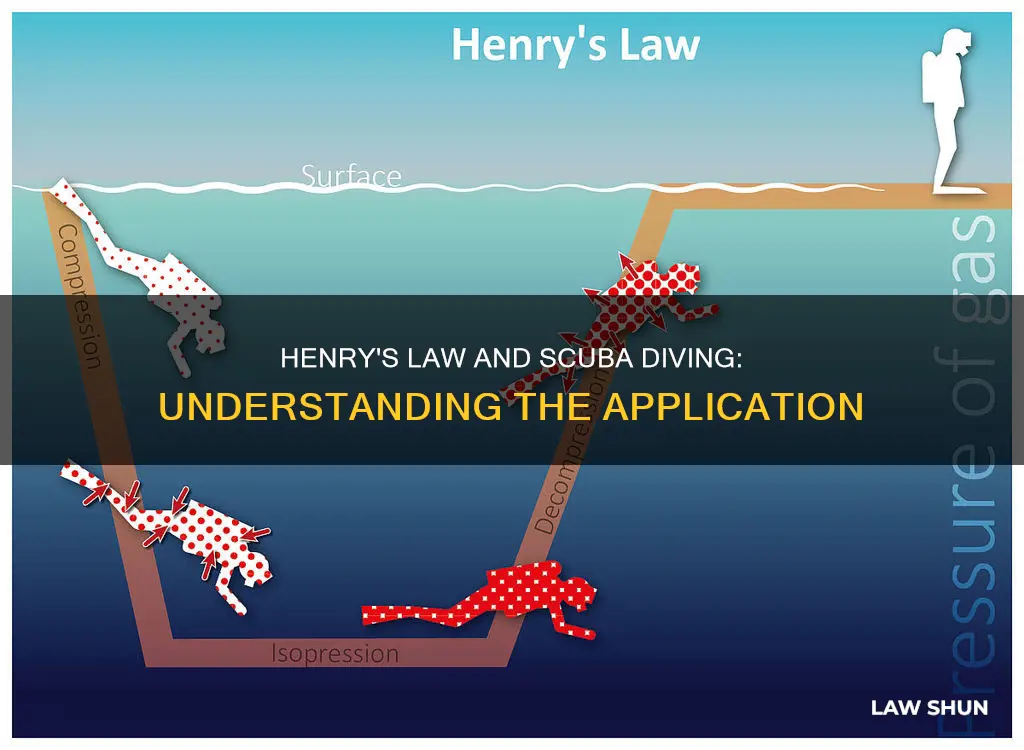
Henry's Law, proposed by English physician and chemist William Henry in 1801, states that the solubility of a gas in a liquid is directly proportional to the pressure of the gas above the liquid. This law is crucial to understanding the risks of scuba diving. As divers descend, the pressure increases, and their bodies absorb more gases, particularly nitrogen, into their blood and tissues. When divers ascend, the pressure decreases, and the nitrogen escapes from the blood, potentially forming dangerous bubbles if the ascent is too rapid. This can lead to decompression sickness, commonly known as the bends, which can be fatal. Therefore, divers must ascend slowly and avoid activities that increase body temperature, such as hot baths or strenuous exercise, after diving to prevent the nitrogen from coming out of solution too rapidly.
| Characteristics | Values |
|---|---|
| Named After | William Henry |
| Date | 1801 or 1803 |
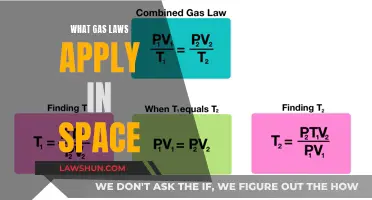
| Formula | P = the partial pressure of the gas, C = the concentration of the gas, K = Henry's Law constant |
| Application to Scuba Diving | Explains how at higher pressure, divers' bodies absorb more gases, particularly nitrogen. |
| Decompression Sickness | Explains how divers may experience decompression sickness ("the bends") if they ascend too quickly, as nitrogen bubbles form in the bloodstream. |
| Temperature | As temperature increases, solubility of gases in liquids decreases. |
What You'll Learn

The solubility of gases in liquids
Firstly, as pressure increases, the solubility of gases in liquids also increases. This means that under higher pressure, a greater amount of gas can be absorbed by a liquid. For scuba divers, this translates to their bodies absorbing more gases at greater depths. The deeper a diver goes, the higher the pressure, and therefore, the greater the amount of nitrogen (and other gases) that will be absorbed into their blood and tissues. This has important implications for decompression, as the absorbed nitrogen needs to be safely released from the body during the ascent.
Secondly, Henry's Law also states that as the temperature increases, the solubility of gases in liquids decreases. A practical example of this is the formation of bubbles when a bottle of soda is opened. The liquid is under pressure, causing the carbon dioxide to be soluble, but when the pressure is released by opening the bottle, the carbon dioxide gas escapes in the form of bubbles. Similarly, during a dive, the nitrogen that has dissolved in the bloodstream under pressure will begin to come out of the solution as the diver ascends and the pressure decreases. If the diver ascends too quickly, the nitrogen may form bubbles, leading to decompression sickness, also known as "the bends". This is why divers must ascend gradually, allowing the nitrogen to dissipate safely.
Henry's Law also explains why divers are advised to avoid hot baths, strenuous activity, and exercise after a dive. The increase in body temperature caused by these activities may cause the nitrogen to become less soluble and increase off-gassing, potentially leading to decompression sickness. Additionally, diving in colder water results in greater nitrogen absorption, which should be considered when planning dive times and depths.
Understanding the solubility of gases in liquids, as described by Henry's Law, is crucial for scuba divers to ensure safe and enjoyable diving experiences. By comprehending the principles of Henry's Law, divers can make informed decisions about their diving practices and avoid potential health risks associated with decompression.
Applying Early to Penn Law: A Smart Decision?
You may want to see also

The release of pressure
Henry's Law is a crucial concept for scuba divers to understand, as it directly impacts their safety and well-being during a dive. The law, named after English physician and chemist William Henry, states that the amount of a gas dissolved in a liquid is directly proportional to the partial pressure of that gas above the liquid, provided the temperature remains constant.
When applied to scuba diving, Henry's Law explains why divers must be cautious when ascending from greater depths. As a diver descends, the pressure increases, and according to Henry's Law, this increased pressure causes a higher concentration of gases, particularly nitrogen, to dissolve into the bloodstream and tissues. This process is known as on-gassing or absorption.
Now, when the diver starts ascending, the pressure decreases, and the dissolved gases start to come out of the solution. This is known as off-gassing. If the diver ascends too quickly, the nitrogen gas can form bubbles in the body, leading to decompression sickness (DCS), commonly known as "the bends." This condition can cause painful and dangerous symptoms, including soreness in the joints, blisters under the skin, and in severe cases, even death.
To avoid decompression sickness, divers are taught to ascend slowly and gradually. This slow ascent allows the nitrogen to dissipate safely without forming bubbles. Additionally, divers are advised to refrain from taking hot baths or engaging in strenuous activities after a dive. According to the second part of Henry's Law, an increase in temperature can cause gases to become less soluble, leading to off-gassing and a potential risk of DCS.
Understanding the release of pressure during the ascent is crucial for scuba divers to prevent dangerous consequences. By following the principles of Henry's Law, divers can ensure a safe and enjoyable diving experience.
The Laws of Physics: Universal or Not?
You may want to see also

Decompression sickness
The two principal factors that control the risk of a diver developing DCS are:
- The rate and duration of gas absorption under pressure. The deeper or longer the dive, the more gas is absorbed into body tissue in higher concentrations than normal (Henry's Law).
- The rate and duration of outgassing on depressurization. The faster the ascent and the shorter the interval between dives, the less time there is for absorbed gas to be offloaded safely through the lungs, causing these gases to come out of solution and form "microbubbles" in the blood.
DCS can produce many symptoms, and its effects may vary from joint pain and rashes to paralysis and death. The severity of symptoms varies from barely noticeable to rapidly fatal. Symptoms of DCS include:
- Skin manifestations, such as itching, usually around the ears, face, neck, arms, and upper torso, and a mottled or marbled skin rash, usually around the shoulders, upper chest, and abdomen, with itching (cutis marmorata).
- Neurological symptoms, such as confusion or memory loss (amnesia), visual abnormalities, unexplained mood or behavior changes, seizures, unconsciousness, ascending weakness or paralysis in the legs, urinary and fecal incontinence, girdling around the abdominal region and/or chest, dizziness, vertigo, nausea, and vomiting.
- Joint pain, with the shoulder being the most commonly involved joint in type I DCS, though any joint may be affected. The pain may be reduced by bending the joint to find a more comfortable position.
DCS is best known as a diving disorder that affects divers who have breathed gas at a higher pressure than the surface pressure, owing to the pressure of the surrounding water. The risk of DCS increases when diving for extended periods or at greater depths without ascending gradually and making the decompression stops needed to slowly reduce the excess pressure of inert gases dissolved in the body.
To prevent the excess formation of bubbles that can lead to DCS, divers limit their ascent rate—the recommended ascent rate is about 10 meters (33 feet) per minute—and follow a decompression schedule as necessary. This schedule may require the diver to ascend to a particular depth and remain at that depth until sufficient inert gas has been eliminated from the body to allow further ascent. Each of these is termed a "decompression stop". Following a decompression schedule does not completely protect against DCS, but it does reduce the probability of its occurrence to a very low level.
The definitive treatment for DCS is recompression and hyperbaric oxygen administered in a recompression chamber. In-water recompression (IWR) with oxygen is a medically recognized option when there is no readily available access to a suitable hyperbaric chamber, and if the symptoms are significant or progressing. Oxygen first aid has been used as an emergency treatment for diving injuries for years, and it is beneficial to give fluids as this helps reduce dehydration.
The Law of Cosines: Beyond Right Triangles
You may want to see also

Gas behaviour at depth
Scuba diving is all about getting gas into your body while you are underwater. However, gas behaviour at depth, under pressure, in your body, and in your equipment is complex. To dive safely and effectively, you need to understand all the behaviours of these gases and how they affect the techniques and practice of scuba diving.
As a diver descends, the pressure increases, and the volume decreases, so the same amount of air takes up less space. This is why a diver's BCD "deflates" as they go deeper. It is not losing air; the air is being compressed into a smaller volume. As a diver ascends, the opposite happens: pressure decreases, and volume increases. A full BCD at depth will become fuller as the diver ascends, which is why air must be released as they rise.
The deeper a diver goes, the higher the partial pressures exhibited by the component gases in their breathing gas. As a diver descends and inhales pressurised air, their bloodstream also absorbs the gaseous particles, including nitrogen. This is an application of Henry's Law, which states that as pressure increases, the solubility of gases in liquids increases. The nitrogen from the compressed air stays in the bloodstream until it is able to escape at a lower pressure through exhalation. However, as the diver is in a highly pressurised environment, the nitrogen can only leave the body when the diver reaches lower pressures, ideally during a gradual ascent.
If a diver ascends too quickly, the nitrogen comes out of the blood too rapidly, forming bubbles, which can cause decompression sickness (DCS), also known as "the bends". This is why divers ascend slowly, to allow the nitrogen to dissipate rather than form bubbles. Henry's Law also explains why divers are advised not to take hot baths or exercise after a dive. The increase in temperature may cause the nitrogen to become less soluble and increase off-gassing, possibly causing DCS.
In summary, Henry's Law is crucial to understanding gas behaviour at depth. It explains how increased pressure at depth causes gases to dissolve in the bloodstream and why a gradual ascent and avoiding activities that increase body temperature after a dive are essential to prevent the dangerous formation of nitrogen bubbles in the body.
Independent Assortment: Monohybrid Crosses and Mendel's Law
You may want to see also

Gas absorption in the body
In the context of scuba diving, this law has significant implications for the absorption of gases into a diver's body. As a diver descends, the pressure increases, and according to Henry's Law, this increased pressure leads to a higher solubility of gases in the bloodstream, muscles, and tissues. This means that gases like nitrogen, which make up 78%-79% of the air we breathe, dissolve in higher concentrations in the body.
The depth of the dive plays a critical role in gas absorption. The deeper a diver goes, the greater the pressure, and consequently, the higher the amount of nitrogen absorbed into the blood and tissues. This is why divers who venture to greater depths, such as 100 feet, are at a higher risk of decompression illness than those who dive to shallower depths, such as 30 feet.
During the descent, the inhaled nitrogen does not pose an immediate problem as it remains dissolved in the body due to the high pressure. However, when the diver starts ascending, the pressure decreases, and the nitrogen begins to come out of solution. If the diver ascends too quickly, the nitrogen can form bubbles in the bloodstream, leading to decompression sickness (DCS), commonly known as "the bends." This condition can cause painful and dangerous symptoms, including soreness in the joints, blisters under the skin, and in severe cases, even death.
To avoid DCS, divers are taught to ascend slowly and gradually, allowing the nitrogen to dissipate safely. Additionally, they are advised to avoid taking hot baths or engaging in strenuous activities after a dive, as increased temperature can also cause the nitrogen to come out of solution, according to the second part of Henry's Law.
Consulting and Public Law 86-272: What's the Verdict?
You may want to see also