The first law of thermodynamics, also known as the law of conservation of energy, states that the total amount of energy in the universe is constant. This means that energy cannot be created or destroyed, only transferred or transformed. This law applies to biological systems, which are open systems that exchange energy with their surroundings. All biological organisms require energy to survive and must obtain it from their surroundings in forms that they can convert into usable energy. Plants, for example, convert sunlight into chemical energy, while humans convert the chemical energy in food into kinetic energy. Understanding how the first law of thermodynamics applies to biological systems is crucial for comprehending the fundamental principles governing energy transfer and transformation in living organisms.
What You'll Learn

Energy transformations in biological systems
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can be transferred and transformed but not created or destroyed. This law applies to biological systems, where energy transformations occur in living organisms as they convert the energy of the sun and food into other types of energy.
ATP synthesis is a critical energy transformation process in both plants and animals. In plants, ATP is synthesized during the day through the absorption of solar energy and subsequent biochemical reactions. In animals, ATP synthesis occurs through the breakdown of glucose and other nutrients derived from food. The energy released during the cleavage of ATP is used to power essential cellular functions, such as DNA replication, cell division, and muscle contraction.
Another example of energy transformation in biological systems is cellular respiration, which occurs in both plant and animal cells. During cellular respiration, glucose and other fuel molecules are broken down in the presence of oxygen to release energy. This process involves a series of enzymatic reactions that ultimately produce ATP and waste products, such as carbon dioxide and water. Cellular respiration provides the energy required for various cellular activities, including active transport and biosynthesis of macromolecules.
Additionally, biological systems also exhibit energy transformations during metabolic processes. Metabolism refers to the set of chemical reactions that occur within an organism to maintain life. These reactions involve the breakdown of complex molecules and the synthesis of new ones, with energy being transferred and transformed at each step. For example, during the breakdown of glucose in cellular respiration, energy is released and captured in the form of ATP, which can then be used to perform mechanical work or fuel other cellular processes.
It is important to note that the second law of thermodynamics also plays a role in biological systems. While energy transformations occur, the total energy within a closed biological system remains constant. However, due to entropy, not all of the available energy is useful to the organism. Some energy is lost as heat or reflected away, as observed in processes like photosynthesis, where plants do not absorb all of the incident light energy.
The Executive Branch: Rule of Law in Action
You may want to see also

The law of conservation of energy
The First Law of Thermodynamics, also known as the law of conservation of energy, is a fundamental principle that governs the behaviour of energy in the universe, including within biological systems. This law states that the total amount of energy in the universe remains constant; it has always been and always will be the same. In other words, energy cannot be created or destroyed; it can only be transferred or transformed from one form to another.
One of the most crucial energy transformations in biology is photosynthesis, which occurs in plants. During photosynthesis, plants absorb light energy from the sun and convert it into chemical energy stored in the form of glucose. This process allows plants to build complex carbohydrates necessary for their growth and development. Additionally, the energy stored in glucose can be released through cellular respiration, providing energy for essential cell functions in both plant and animal organisms.
Another important example of energy transformation in biology is the conversion of chemical energy in food into kinetic energy in living beings. This transformation enables humans to perform tasks such as riding a bicycle, which requires energy for movement. Similarly, the chemical energy stored in organic molecules like sugars and fats is transformed through cellular chemical reactions into energy within molecules of adenosine triphosphate (ATP). ATP molecules provide easily accessible energy for essential cellular functions, including building complex molecules, transporting materials, muscle contraction, and reproduction.
Texting Laws: Do They Apply When Your Vehicle Is Parked?
You may want to see also

Metabolism in biological organisms
The laws of thermodynamics are important unifying principles of biology, governing the chemical processes (metabolism) in all biological organisms. The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy can neither be created nor destroyed. This law applies to biological systems, including the metabolism of organisms.
Metabolism is the process by which energy is derived from food and other sources and converted into energy that can be used for essential biological processes. This is a fundamental process for all living organisms, and it is governed by the laws of thermodynamics. The first law states that the total amount of energy in the universe remains constant; it can be transferred or transformed but not created or destroyed. This means that the energy within an organism is not consumed but rather transformed from one form to another.
For example, plants convert sunlight into chemical energy through photosynthesis. This energy is stored in the form of glucose, which is then used to form complex carbohydrates necessary for building plant mass. This process demonstrates the first law in action, as the energy from the sun is transformed into chemical energy within the plant.
In animals, energy is derived from consuming plants or other animals, and this energy is then transformed through cellular respiration. This process releases the energy stored in carbohydrates, lipids, and other macromolecules, which is then used for essential cellular functions. The energy derived from food is stored in molecules such as sugars and fats, which are then transformed through a series of cellular reactions into energy within ATP (adenosine triphosphate) molecules. ATP provides easily accessible energy for essential cellular processes, including building complex molecules, transporting materials, creating movement through muscle fibre contraction, and reproduction.
The work of scientists such as Erwin S. Bauer, Hans Krebs, Hans Kornberg, and Boris Dobroborsky has contributed significantly to our understanding of the role of thermodynamics in biological systems, including metabolism. Their research has elucidated the ways in which living organisms utilise and transform energy to maintain their highly ordered state, adapting to their environment through phenotypic adaptation.
HIPAA Laws: Paying Bills, Sharing Info, and Privacy
You may want to see also

The challenge of energy acquisition
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can be transferred and transformed but not created or destroyed. This total amount of energy in the universe is constant.
For example, plants perform one of the most biologically useful energy transformations: converting the energy of sunlight into the chemical energy stored within organic molecules. This is achieved through a series of cellular chemical reactions that transform the chemical energy stored within organic molecules such as sugars and fats into energy within molecules of ATP. This energy is easily accessible and allows cells to perform a variety of functions, including building complex molecules, transporting materials, creating movement, and reproduction.
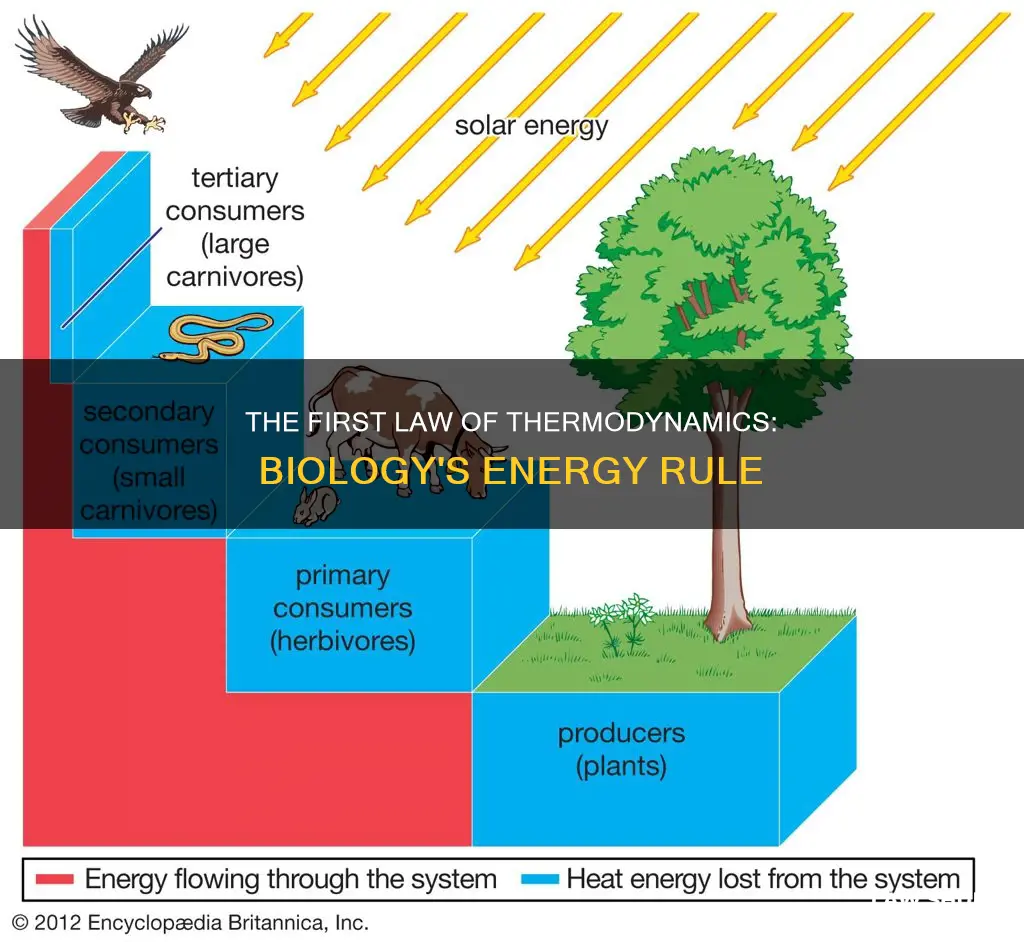
Animals, on the other hand, cannot generate energy directly from sunlight and must consume plants or other animals for energy. The higher an organism is on the food chain, the less available energy it receives from its food sources, as much of this energy is lost during metabolic processes. This is why there are more producers than consumers in an ecosystem.
Living systems require a constant energy input to maintain their highly ordered state, and some energy is always lost to the surroundings or transformed during these processes.
HIPAA Laws: Do They Extend to Military Personnel?
You may want to see also

Energy transfer and entropy
The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that the total amount of energy in the universe remains constant. In other words, energy cannot be created or destroyed, only transferred or transformed. This law applies to all biological systems, which are open systems that constantly exchange energy with their surroundings.
Biological organisms require energy to survive, and they obtain this energy by consuming energy-storing molecules and converting them into usable energy through a series of cellular chemical reactions. For example, plants convert sunlight into chemical energy through photosynthesis, storing it in the form of glucose. Animals, on the other hand, cannot generate energy directly from sunlight and must consume plants or other animals to obtain energy.
The energy obtained by biological organisms is used for various cellular functions, such as building complex molecules, transporting materials, creating movement, and reproduction. These processes involve the transformation of energy from one form to another, such as the conversion of chemical energy in food into kinetic energy for movement.
However, the Second Law of Thermodynamics explains that energy transfers are never completely efficient. With every energy transfer, some amount of energy is lost in a form that cannot be used, usually as heat energy. This loss of energy contributes to an increase in disorder or entropy in the system. Entropy is a measure of randomness or disorder in a system, and it increases as energy is transferred or transformed.
Living organisms, therefore, face the challenge of constantly acquiring and converting energy to maintain their highly ordered state and perform necessary functions. While the total energy in a closed system, such as the universe, remains constant, the usable energy available to an organism decreases with each transfer and transformation, due to the increase in entropy. This loss of usable energy with each energy transfer contributes to the overall increase in entropy in the universe.
Affinity Laws: Open Loop Systems and Their Limitations
You may want to see also
Frequently asked questions
The First Law of Thermodynamics states that the total amount of energy in the universe is constant. In other words, the amount of energy in the universe remains the same. Energy can be transferred or transformed but cannot be created or destroyed.
The First Law of Thermodynamics applies to biological systems as they are open systems that exchange energy with their surroundings. Living organisms obtain energy from their surroundings and transform it into usable energy to perform various functions.
One of the most important energy transformations in biology is photosynthesis, where plants convert sunlight into chemical energy stored in organic molecules like glucose.
Adenosine triphosphate (ATP) is synthesized from food and acts as the main source of energy for living organisms. It is easily accessible energy that cells use to perform functions such as building complex molecules, transporting materials, and creating movement.
The First Law of Thermodynamics states that energy in a closed system remains constant, while the Second Law of Thermodynamics discusses the concept of entropy, stating that energy transfers are never completely efficient and always result in a loss of usable energy.







