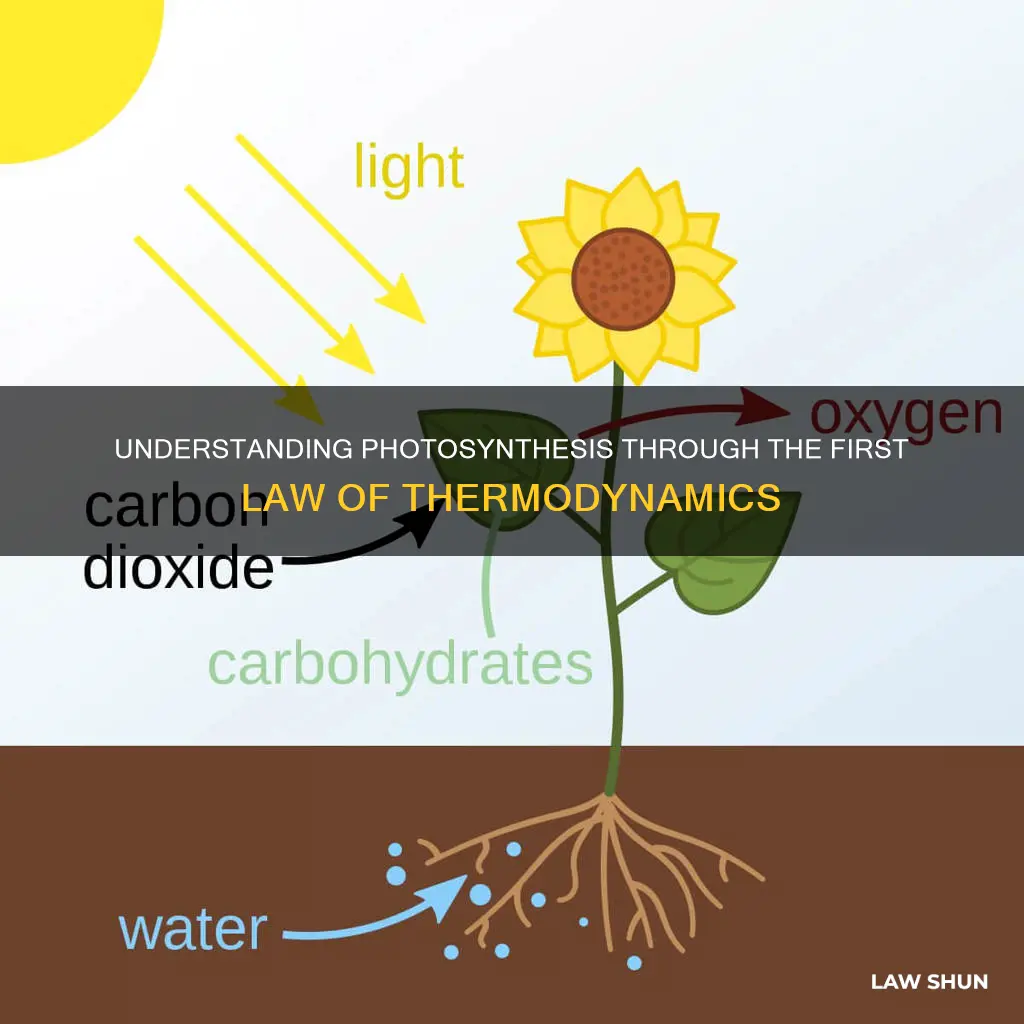
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed. This principle applies to biological systems, including photosynthesis, where energy transformation plays a crucial role. In the process of photosynthesis, plants absorb solar energy and convert it into molecular potential energy in the form of sugar, which is a type of chemical energy. This conversion process aligns with the first law of thermodynamics, as the energy from the sun is not created or destroyed but transformed into a different form.
What You'll Learn

Energy conversion in photosynthesis
The First Law of Thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. This principle is clearly illustrated in the process of photosynthesis, where plants convert solar energy into chemical energy.
During the day, plants absorb solar energy from the sun. This energy is then transformed into molecular potential energy in the form of sugar, which is a type of chemical energy. This process is known as photosynthesis, and it is essential for plants to create their food.
The light phase of photosynthesis, which occurs in the chloroplast membranes, holds the key to understanding solar energy conversion into chemical energy. Chloroplast membranes are composed of lipids and proteins, and by studying their molecular architecture, scientists can gain insights into how plants convert solar energy. This knowledge is crucial for developing model systems that can simulate the photosynthetic process and explore its potential for solar fuel production.
The chemical energy stored in the form of glucose during photosynthesis is not only used by plants to build complex carbohydrates necessary for their growth but can also be released through cellular respiration. This process allows both plant and animal organisms to access the energy stored in carbohydrates, lipids, and other macromolecules through the production of ATP. This released energy is vital for performing essential cell functions, such as DNA replication, cell movement, and apoptosis.
In summary, the energy conversion in photosynthesis directly aligns with the First Law of Thermodynamics. Solar energy, or light energy, is converted into chemical energy, demonstrating that energy can be transformed from one form to another while remaining conserved in a closed system.
Who Are Mandatory Reporters? Dependednt Care and the Law
You may want to see also

The first law of thermodynamics and the conservation of energy
The first law of thermodynamics, also known as the law of conservation of energy, is a fundamental principle that governs the behaviour of energy in the universe. According to this law, energy cannot be created or destroyed; it can only be converted from one form to another. This law applies to all systems, including biological processes such as photosynthesis.
Photosynthesis is a perfect example of the first law of thermodynamics in action. During photosynthesis, plants absorb sunlight, which is a form of solar energy. Through a series of complex reactions, plants convert this solar energy into chemical energy in the form of glucose, also known as sugar. This process demonstrates the law of conservation of energy because the energy from the sun is not being created or destroyed; it is simply being transformed into a different form.
The chemical energy stored in glucose is essential for the plant's growth and metabolism. It is used to form complex carbohydrates, which are necessary for building plant mass and carrying out various cellular functions. Additionally, the energy in glucose can be released through cellular respiration, allowing both plant and animal organisms to access and utilise this stored energy.
The first law of thermodynamics highlights the importance of energy conservation and transformation. In the context of photosynthesis, it shows how plants harness solar energy and convert it into a form that can be stored and used to sustain life. This process is crucial for the survival of plants and serves as the foundation for energy transfer throughout the food chain.
Moreover, the first law of thermodynamics also applies to other biological processes beyond photosynthesis. For instance, human metabolism involves the conversion of food into heat, work, and stored fat. This process demonstrates the law of conservation of energy, as the energy from food is neither created nor destroyed but instead undergoes transformation to support various bodily functions.
Labor Law 200: Who is Covered and Who is Exempt?
You may want to see also

Photosynthesis as an irreversible process
The First Law of Thermodynamics, also known as the Law of Conservation of Energy, states that energy cannot be created or destroyed but can only change from one form to another. In the context of photosynthesis, this means that the solar energy absorbed by plants cannot be generated or eliminated but can be transformed. During photosynthesis, plants convert solar energy or light energy into chemical energy in the form of sugar. This process demonstrates the First Law of Thermodynamics in action, as the energy is conserved and changed from one form to another.
Photosynthesis is an irreversible process, meaning that the products formed during the reaction do not revert to their original state as reactants. This irreversibility is due to the nature of the system in which photosynthesis occurs, which is an open system. In an open system, the products of one reaction, such as oxygen, can quickly leave the plant through its stomata (pores in the leaves). Additionally, the enzymes present in the plant cells only catalyse the forward reaction, and the activation energy required for the reverse reaction is too high in the absence of a suitable enzyme.
Furthermore, the synthesis of glucose from carbon dioxide and water, which is a crucial step in photosynthesis, is a highly unfavourable process. This is because the reaction is endothermic, requiring an input of heat energy, and it also results in a decrease in entropy or disorder within the system. The overall process of photosynthesis is endothermic, with a positive Gibbs free energy (∆G > 0), indicating that it is non-spontaneous and irreversible.
The irreversibility of photosynthesis is in contrast to other processes, such as the inflation of a balloon, which is a reversible change. When a balloon is inflated, it undergoes a change in shape and size, but when the air is allowed to escape, it returns to its original state. However, in the case of photosynthesis, once solar energy is converted into chemical energy, the reaction does not spontaneously reverse, and the products do not transform back into their original reactants.
The irreversible nature of photosynthesis has significant implications for the field of biology. It highlights the importance of energy conversion and conservation in biological systems, as the energy absorbed and transformed during photosynthesis is essential for the survival and growth of plants. Additionally, the irreversibility of the process contributes to the overall complexity and step-wise nature of photosynthesis, showcasing the intricate mechanisms that plants employ to convert solar energy into usable chemical energy.
Understanding ADA Compliance for Cell Phones
You may want to see also

The role of photons in photosynthesis
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed. It can, however, be transformed from one form to another. This principle is clearly evident in the process of photosynthesis, where plants convert solar energy into chemical energy.
The photosynthetic pigments are organised into photocenters or antenna complexes, each containing hundreds of pigment molecules. These antenna complexes act as light-harvesting structures, maximising the plant's ability to capture photons. The absorbed photons transfer their energy to neighbouring molecules through successive fluorescence events until they reach the reaction centre.
At the reaction centre, the energy is used to transfer an energetic electron to an electron acceptor molecule. This initiates an electron transport chain, where high-energy electrons are transferred through a series of membrane carriers, facilitating the synthesis of ATP and NADPH. The ATP and NADPH then drive the dark reactions of photosynthesis, where glucose is synthesised from CO2 and H2O.
In summary, photons play a crucial role in photosynthesis by providing the initial energy input that is captured by pigments and converted into chemical energy through a series of electron transfers. This ultimately leads to the synthesis of glucose, which is essential for plant growth and serves as the primary source of metabolic energy for all biological systems.
Obtaining a Law License in Texas: A Guide
You may want to see also

The efficiency of photosynthesis
> 6 H2O + 6 CO2 + energy → C6H12O6 + 6 O2
Where C6H12O6 is glucose, which is subsequently transformed into other sugars, starches, cellulose, and lignin. The efficiency of photosynthesis depends on how light energy is defined and the type of light used. It takes eight, or perhaps ten or more, photons to use one molecule of CO2. The nominal efficiency of this process is 30%. However, the theoretical maximum efficiency of solar energy conversion is approximately 11%. In reality, plants do not absorb all incoming sunlight due to reflection, respiration requirements, and the need for optimal solar radiation levels. This results in a maximum overall photosynthetic efficiency of 3 to 6% of total solar radiation.
Efforts to increase photosynthetic efficiency include incorporating pigments like chlorophyll d and f, which can absorb far-red light, into the photosynthetic machinery of higher plants. By adapting these pigments, it is hoped that plants can be engineered to utilise a wider range of the light spectrum, leading to increased growth rates and biomass production. Another approach is to expand the absorption in the green wavelengths in plants by using chlorophyll c, a pigment found in marine algae with blue-green absorption.
Antique Firearms: Concealed Carry Law Exemptions?
You may want to see also
Frequently asked questions
The first law of thermodynamics, also known as the law of conservation of energy, states that energy can neither be created nor destroyed. This is relevant to photosynthesis as energy is not being created or destroyed during the process. Instead, solar energy is converted into chemical energy in the form of sugar.
The first law of thermodynamics states that "energy can neither be created nor destroyed, but it can be transformed from one form to another."
During photosynthesis, plants absorb solar energy from the sun and convert it into molecular potential energy in the form of sugar, which is a type of chemical energy. This chemical energy is then stored in the plant as glucose.