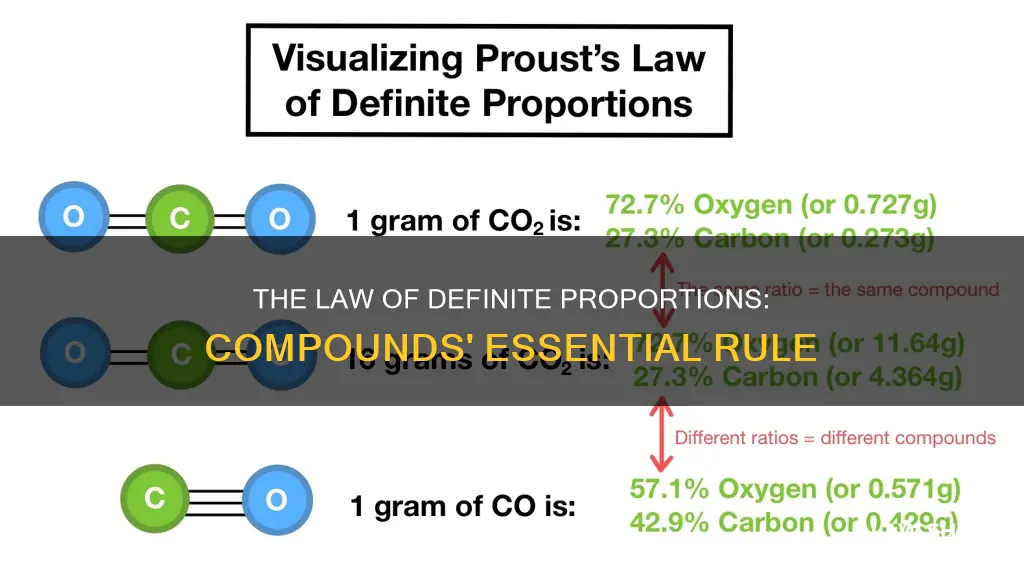
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its constituent elements in a fixed ratio by mass. This means that the composition of a compound is independent of its source or method of preparation. For example, in water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9, and this ratio is consistent across all samples of water. This law was first formulated by French chemist Joseph Proust in 1794 from his work on sulphides, metallic oxides, and sulfates.
What You'll Learn

The law applies to compounds, not elements
The law of definite proportions, also known as Proust's Law or the law of constant composition, applies to compounds, not elements. This means that a compound will always contain the same proportion of elements by mass, regardless of the source of the elements or how the compound is prepared.
For example, in water, the mass of oxygen must always have the same ratio to the mass of hydrogen. In any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio is always the same, no matter where the water sample came from or how it was prepared.
The law of definite proportions was first proposed by French chemist Joseph Proust in 1794, based on the study of combustion. However, it was disputed by other chemists at the time, such as Claude Louis Berthollet, who argued that elements could combine in any proportion. It was later supported by the atomic theory of John Dalton, who explained that matter consists of discrete atoms, with one type of atom for each element, and that compounds are made of combinations of different types of atoms in fixed proportions.
While the law of definite proportions is a useful concept in chemistry, it is important to note that there are exceptions. Some compounds, such as wustite, are non-stoichiometric, meaning their elemental composition can vary from sample to sample. Additionally, the isotopic composition of an element can vary depending on its source, which can affect the mass of a pure stoichiometric compound.
Grouping Law and Multiplication: How Are They Connected?
You may want to see also

The ratio of elements in a compound is fixed by mass
The law of definite proportions, also known as Proust's Law or the law of constant composition, states that a given chemical compound will always contain its component elements in a fixed ratio by mass. This means that the composition of a compound is always the same, regardless of its source or method of preparation.
For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio is always the same, no matter where the sample of water came from or how it was prepared.
The law of definite proportions was first proposed by French chemist Joseph Proust in 1794, based on the study of combustion. However, it was contested by fellow French chemist Claude Louis Berthollet, who argued that elements could combine in any proportion. It was later supported by English chemist John Dalton's atomic theory, which explained that matter consists of discrete atoms, with one type of atom for each element, and that compounds are made of combinations of different types of atoms in fixed proportions.
While the law of definite proportions forms the basis of stoichiometry, it is important to note that there are exceptions to this rule. Some compounds, known as non-stoichiometric compounds, have elemental compositions that vary from sample to sample. Additionally, the isotopic composition of an element can vary depending on its source, which can affect the mass of a pure stoichiometric compound.
Natural Law Theory's Take on the Trolley Problem
You may want to see also

The origin of the elements does not affect the ratio
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and this ratio does not depend on the source of the compound or the method of its preparation. This means that the origin of the elements does not affect the ratio in which they combine to form a compound.
For example, in water, the ratio of hydrogen to oxygen by mass is always 1:8, or 11.19% hydrogen to 88.81% oxygen. This ratio is consistent regardless of the source of the water or how it was prepared. Whether the water was obtained from a river or a tap, the ratio of hydrogen to oxygen remains the same.
Another example is carbon dioxide, which always consists of one atom of carbon and two atoms of oxygen. This 1:2 ratio is consistent regardless of how the carbon dioxide was produced, such as through the burning of materials.
The law of definite proportions was first formulated by French chemist Joseph Proust in 1794 based on his work on sulphides, metallic oxides, and sulfates. It was later supported by Scottish chemist Thomas Thomson and formulated into chemical atomic theory by English chemist John Dalton in 1808.
Moore's Law and Solar Cells: A Relevant Relationship?
You may want to see also

The method of preparation does not affect the ratio
The law of definite proportions, also known as Proust's Law or the law of constant composition, states that a compound will always contain the same proportion of elements by mass. This means that the method of preparation does not affect the ratio of elements in a compound.
The law was first observed by English theologian and chemist Joseph Priestley and French nobleman and chemist Antoine Lavoisier in 1794, and was later stated by Joseph Proust in 1797. Proust's law observes that a given chemical compound will always contain its component elements in a fixed ratio by mass, regardless of its source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9.
The law of definite proportions contributed to and was placed on a firm theoretical basis by the atomic theory promoted by John Dalton from 1803. This theory explained that matter consists of discrete atoms, with one type of atom for each element, and that compounds are made of combinations of different types of atoms in fixed proportions.
The law of definite proportions is an important concept in stoichiometry, which is the study of the quantitative relationships between the reactants and products in chemical reactions. It allows us to determine the ratios of masses of different elements in a compound, and to predict the effects of different chemical processes. For example, knowing the exact composition of carbon dioxide, which is one carbon atom and two oxygen atoms, allows us to make predictions about the effects of different chemical processes, such as the amount of carbon dioxide produced by burning wood or fossil fuels.
HIPAA Laws: Who Is Bound and Who Is Exempt?
You may want to see also

The law was first observed by Joseph Proust in 1797
The law of definite proportions, also known as Proust's Law, was first observed by Joseph Proust in 1797. However, the original observation was made by English theologian and chemist Joseph Priestly and French nobleman and chemist Antoine Lavoisier, who centred their work on the process of combustion.
Proust's Law states that a compound always contains exactly the same proportion of elements by mass. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. This ratio is always the same, no matter the source of the water or how it was prepared.
Proust's Law was a novel idea at the time, as the concept of a chemical compound had not yet been fully developed. It was initially controversial and was opposed by Proust's fellow Frenchman, Claude Louis Berthollet, who argued that elements could combine in any proportion.
The law of definite proportions was placed on a firm theoretical basis by John Dalton's atomic theory, which was promoted from 1803. This theory explained that matter consists of discrete atoms, with one type of atom per element, and that compounds are made of different types of atoms in fixed proportions.
The Law of Conservation: Universal or Not?
You may want to see also