The law of definite proportions, also known as Proust's law or the law of constant composition, states that a compound will always contain the same proportion of elements by mass. This law applies to hydrates, which are salts that contain water. Hydrates always maintain a set ratio of constituent elements, with the water chemically combined with the compound in a definite ratio. For example, in magnesium sulfate heptahydrate (MgSO₄·7H₂O), the ratio of water to salt is always 1.047:1. This ratio remains constant regardless of the amount or source of the compound.
What You'll Learn
- Hydrates are water-containing salts
- The law of definite proportions applies to both liquid and ionic hydrates
- The ratio of elements in a hydrate does not depend on the amount or source of the compound
- The formula for an ionic hydrate combines the formula of the ionic compound and the formula for water
- The law of definite proportions is also known as Proust's law or the law of constant composition

Hydrates are water-containing salts
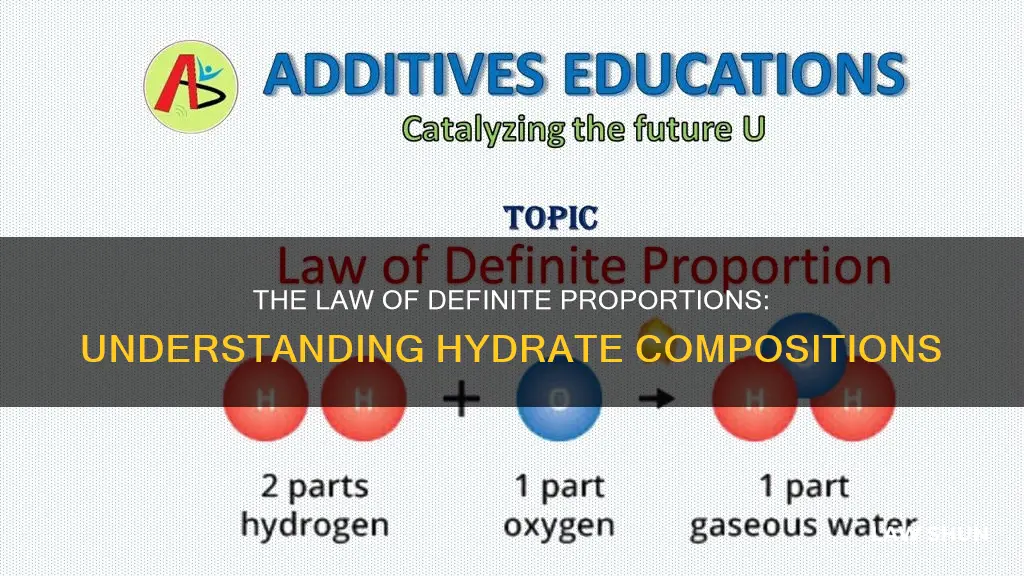
For example, in water, the ratio of oxygen to hydrogen is always 8:2 by mass, giving it the chemical formula H2O. This ratio remains constant, no matter the source of the water.
Hydrates, as water-containing salts, also follow this law. For instance, magnesium sulphate heptahydrate, with the formula MgSO4·7H2O, always contains a fixed ratio of water to salt. In this case, for every 120.4 g of MgSO4, there are 126.1 g of H2O, giving a ratio of approximately 1:1.047.
Similarly, copper sulphate pentahydrate, with the formula CuSO4·5H2O, contains five water molecules for every unit of the ionic compound. When heated, the water is removed, yielding copper (II) sulphate anhydrous and five water molecules. The ratio of oxygen to hydrogen in each water molecule remains at 16:2, in accordance with the law of definite proportions.
Thus, the law of definite proportions tells us that hydrates, as chemical compounds, will always contain a definite and constant ratio of salt to water.
Alcohol Laws: Private Property Exemption?
You may want to see also

The law of definite proportions applies to both liquid and ionic hydrates
The law of definite proportions, also known as the law of constant composition, states that a compound will always contain the same elements in the same proportion by mass. This law applies to both liquid and ionic hydrates.
Hydrates are compounds that contain water, usually in the form of H2O molecules. The chemical state of the water varies across different classes of hydrates. The best-known hydrates are crystalline solids that lose their fundamental structure when the water is removed.
Liquid hydrates, such as pure water, always contain the same proportion of elements by mass. For example, the ratio of oxygen to hydrogen in water will always be $16:2$, giving it the chemical formula ${{\text{H}}_{\text{2}}}{\text{O}}$.
Ionic hydrates, on the other hand, are inorganic salts that contain water molecules combined in a definite ratio as an integral part of their crystal structure. These hydrates are typically bound to a metal center or have crystallized with the metal complex. The formula for an ionic hydrate is written by combining the formula of the ionic compound with the formula for water, indicating the number of water molecules attached. For example, the formula for copper sulphate pentahydrate, which contains five water molecules, is ${\text{CuS}}{{\text{O}}_{\text{4}}}{\text{.5}}{{\text{H}}_{\text{2}}}{\text{O}}$.
The law of definite proportions holds true for both liquid and ionic hydrates because, regardless of their source or amount, they always maintain a set ratio of constituent elements. This means that whether you take water from a lake, a tap, or a bottle, it will always have the same ratio of oxygen to hydrogen. Similarly, the mass ratio of elements in an ionic hydrate, such as copper sulphate pentahydrate, remains constant regardless of the amount or source of the compound.
HIPAA Laws and the President: Who's Exempt?
You may want to see also

The ratio of elements in a hydrate does not depend on the amount or source of the compound
The law of definite proportions, also known as the law of constant composition, states that a compound will always contain the same proportion of elements by mass. This law applies to both liquid and ionic hydrates, which are salts with a certain amount of water included in their structure.
Hydrates, such as magnesium sulfate heptahydrate (MgSO₄·7H₂O), always maintain a fixed ratio of salt to water. In the case of MgSO₄·7H₂O, the ratio of H₂O to MgSO₄ is approximately 1.047:1. This ratio remains constant, regardless of the amount or origin of the compound.
The law of definite proportions was first observed by English theologian and chemist Joseph Priestley and French nobleman and chemist Antoine Lavoisier in the late 18th century. It was later stated by Joseph Proust in 1794 and contributed to the development of atomic theory by John Dalton in 1803.
Legal Age Laws: Residents' Rights and Restrictions
You may want to see also

The formula for an ionic hydrate combines the formula of the ionic compound and the formula for water
The formula for a hydrate always contains the same proportion of salt and water by mass, and this ratio does not change depending on the amount or source of the compound. For example, in the above copper sulphate pentahydrate, the ratio of copper sulphate to water is approximately 1:2.
The law of definite proportions, which states that a compound will always contain the same proportions of elements by mass, applies to hydrates. This is true for both liquid and ionic hydrates, which always maintain a set ratio of constituent elements. For instance, in water, the ratio of oxygen to hydrogen is always 16:2, giving it the formula {H2O}.
The Discovery Law: Hearing Application Explained
You may want to see also

The law of definite proportions is also known as Proust's law or the law of constant composition
The law of definite proportions, also known as Proust's Law or the law of constant composition, is a fundamental concept in chemistry. It was formulated by French chemist Joseph-Louis Proust in 1797, although it was first proposed in 1794 by English theologian and chemist Joseph Priestly and French chemist Antoine Lavoisier. The law states that a given chemical compound will always contain its constituent elements in a fixed ratio by mass, regardless of its source or method of preparation. In other words, it doesn't matter where the compound comes from or how it was made; the ratio of its elements will always be the same.
For example, in any sample of pure water, oxygen will always make up about 8/9 of its mass, while hydrogen will make up the remaining 1/9. This can be expressed by the chemical formula H2O, where the relative number of hydrogen and oxygen atoms is constant.
The law of definite proportions is particularly relevant to hydrates, which are salts that contain water as part of their structure. Hydrates always contain water in a fixed amount, and the water is chemically combined with the compound in a definite ratio. For instance, the formula for magnesium sulfate heptahydrate is MgSO4·7H2O, indicating that it contains seven water molecules for every unit of the ionic compound. This means that the ratio of H2O to MgSO4 is always 1.047/1.
The law of definite proportions is a cornerstone of stoichiometry, which involves calculating the relative quantities of reactants and products in chemical reactions. It also laid the foundation for John Dalton's atomic theory, which explained that matter consists of discrete atoms, with each element having a unique type of atom, and that compounds are formed by combining different types of atoms in fixed proportions.
Florida Family Law: Retroactive Rules and Procedures?
You may want to see also
Frequently asked questions
The law of definite proportions, also known as Proust's law or the law of constant composition, states that a compound will always contain the same proportion of elements by mass.
Hydrates are salts that contain a certain amount of water. The water is chemically combined with the compound in a definite ratio. For example, in magnesium sulfate heptahydrate (MgSO₄·7H₂O), the ratio of H₂O to MgSO₄ is always 1.047/1.
To calculate the ratio of elements in a hydrate, you need to know the formula of the hydrate and the molar masses of its constituent elements. For example, in copper sulfate pentahydrate (CuSO₄·5H₂O), the molar mass of CuSO₄ is 159.6 g/mol, and the molar mass of five water molecules is 90 g/mol. Therefore, the ratio of copper sulfate to water is approximately 2:1.







