The law of conservation of energy is a fundamental principle in science, stating that energy cannot be created or destroyed, only transformed from one form to another. This law applies to all systems, including living organisms, and is of critical importance in physics and chemistry. In biology, the law of conservation of energy helps us understand how energy flows through ecosystems, with energy from the Sun being captured and converted by plants and algae, and then passed on to consumers at higher levels of the food chain. This law also applies to the chemical reactions that occur within living organisms, where energy is stored within chemical bonds and released when molecules are broken down.
What You'll Learn

The law of conservation of energy and mass-energy equivalence
The law of conservation of energy states that energy cannot be created or destroyed, only converted from one form to another. This principle applies to both isolated and closed systems. In an isolated system, the total energy remains constant over time. In a closed system, the total amount of energy within the system can only change if energy enters or leaves the system.
This law has been expressed by the equation:
[math]U_{T} = U_{i} + W + Q [/math]
Where:
- [math]U_T] is the total internal energy of a system
- [math]U_i] is the initial internal energy of a system
- [math]W] is the work done by or on the system
- [math]Q] is the heat added to or removed from the system
The change in internal energy can be determined using the equation:
[math]\Delta U = W + Q [/math]
This is also a statement of the first law of thermodynamics.
At the beginning of the 20th century, Einstein discovered that mass is also a form of energy, known as mass-energy equivalence. This discovery radically changed the notion of mass, showing that it is equivalent and convertible to energy. This relationship is described by the famous formula:
[math]E = mc^{2} [/math]
Where:
- [math]E] is the amount of energy in an object or system
- [math]m] is the mass of the object or system
- [math]c] is the speed of light
This formula implies that even a small amount of matter contains a large amount of energy. For example, during nuclear fission or fusion, some of the mass of the nucleus is converted into huge amounts of energy. Thus, mass-energy equivalence demonstrates that the conservation of energy is equivalent to the conservation of mass.
HIPAA and Workers' Comp: Understanding Privacy Law Compliance
You may want to see also

Energy cannot be created or destroyed
The law of conservation of energy is one of the most important principles in science, and it applies to biology as it does to physics and chemistry. The law states that energy cannot be created or destroyed; it can only be converted from one form to another. This means that the total energy in a closed system remains constant over time.
The law of conservation of energy can be applied to all forms of energy, including kinetic and potential energy. Kinetic energy is the energy associated with the motion of an object. The faster an object is moving, the greater its kinetic energy. Potential energy, on the other hand, is the energy stored in an object or system. For example, a book on a high shelf has potential energy due to its vertical position.
Energy conversion occurs when energy is transferred from one location to another and changes form. For instance, when a stick of dynamite explodes, chemical energy is converted to kinetic energy. The total energy before and after the explosion remains the same.
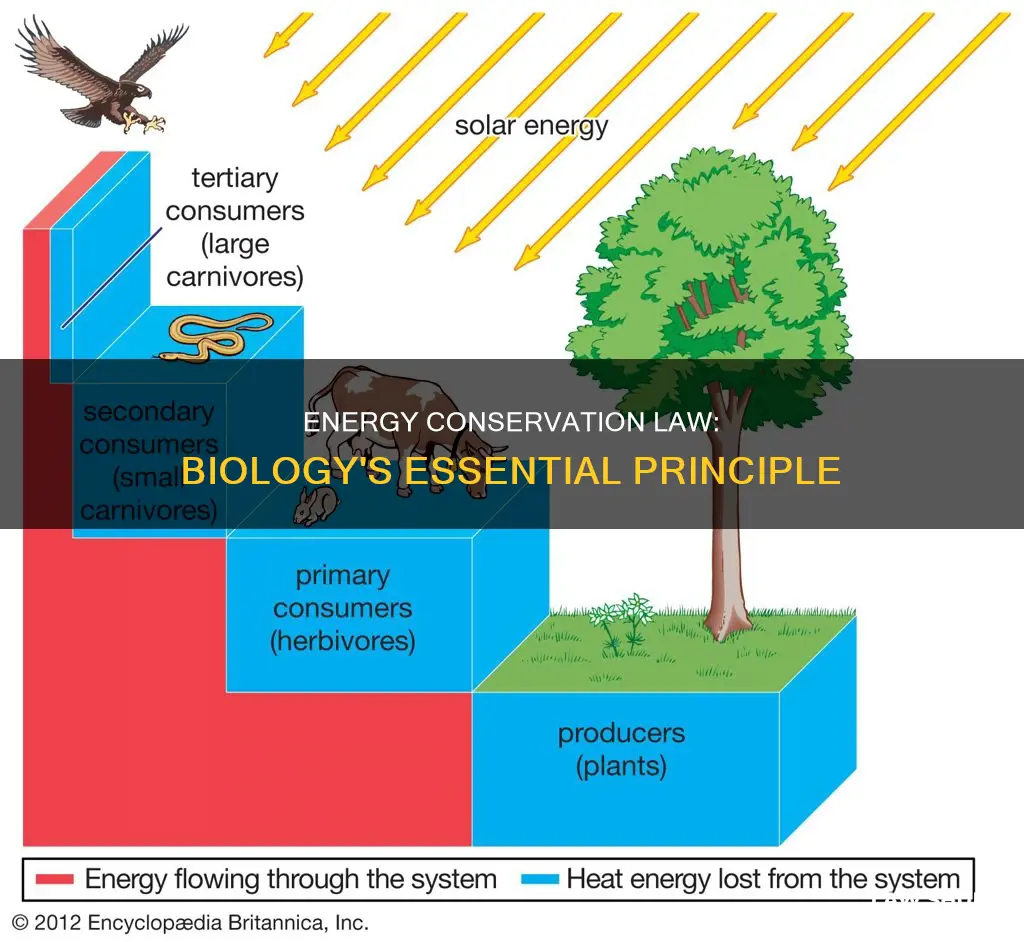
In biology, the law of conservation of energy applies to living systems, where energy changes form as it flows through an ecosystem, but the total amount of energy in the system remains constant. The main source of energy in most ecosystems is light energy from the Sun, which is captured by producers such as plants and algae during photosynthesis. This light energy is converted into chemical energy, which is then passed to consumers at higher levels on the food chain.
Overall, the law of conservation of energy states that energy cannot be created or destroyed, only transformed, and this principle holds true across various scientific disciplines, including biology.
Gauss's Law: Understanding Real-World Applications
You may want to see also

Energy can be converted from one form to another
The law of conservation of energy is one of the most important principles in science, and it applies to biology as well. The law states that energy can neither be created nor destroyed but can only be converted from one form to another. This means that the total amount of energy in a closed system remains constant over time.
Energy conversion can be observed in various biological contexts. For example, in living systems, energy changes form as it flows through an ecosystem, but the total amount of energy in the system remains constant. The main source of energy in most ecosystems is light energy from the Sun, which is captured by producers (plants and algae) during photosynthesis. These organisms convert light energy into chemical energy, which is then passed on to consumers at higher levels of the food chain. As consumers use this energy for life activities, it is converted into mechanical or other forms of energy. Most of the energy transferred to each level of the food chain is "lost" as heat to the environment, but it is not lost from the ecosystem as a whole.
Another example of energy conversion in biology is the process of cellular respiration, where the chemical energy stored in glucose (produced during photosynthesis) is converted back into thermal energy and light energy as by-products, with some energy also being converted into mechanical energy to perform work, such as muscle movement.
Energy conversion also occurs in human-made systems. For instance, in a torch, the chemical energy of batteries is converted into electrical energy, which is then transformed into light and heat energy. Similarly, in a loudspeaker, electrical energy is converted into sound energy, and in a generator, mechanical energy is converted into electrical energy.
These examples illustrate how the law of conservation of energy applies to various biological and human-made systems, demonstrating the ability to convert energy from one form to another while maintaining the total energy within a closed system.
Stark Law and Physical Therapists: Understanding the Legal Boundaries
You may want to see also

The total energy of a closed system remains constant
The law of conservation of energy is a foundational principle in physics, supported by extensive experimental evidence. This law states that energy cannot be created or destroyed in a closed system; it can only be transformed or transferred from one form to another.
A closed system is one that does not exchange energy with its surroundings. This means that all energy transfers or transformations occur within the system itself, maintaining the total energy constant. For example, when you drop a ball, its potential energy converts to kinetic energy as it falls, but the total energy remains unchanged.
The amount of energy in any system is determined by the following equation:
UT = Ui + W + Q
Where:
- UT is the total energy of a system
- Ui is the initial energy of a system
- Q is the heat added or removed from the system
- W is the work done by or on the system
The change in the internal energy of the system can be calculated using the equation:
ΔU = W + Q
This principle is essential for analyzing various physical systems and is applied in many areas of physics, including mechanics, thermodynamics, chemistry, and nuclear physics.
The law of conservation of energy also has practical implications. For example, in mechanical systems, engines convert chemical energy into kinetic energy, and in electrical circuits, electric energy transforms into various forms such as heat or light, but the total energy remains unchanged.
The concept of energy conservation was developed during the 19th century, with key figures such as James Prescott Joule contributing significantly to our understanding of energy transformations.
In summary, the total energy of a closed system remains constant due to the law of conservation of energy. This law is a fundamental principle in physics, and it helps us analyze how systems work and predict their behavior.
Understanding Landlord Laws: Paperwork or Not?
You may want to see also

The law of conservation of energy applies to living systems
The law of conservation of energy is a fundamental principle in science, and it applies to living systems just as it does to physical and chemical systems. This law states that energy cannot be created or destroyed, only transformed from one form to another. In other words, the total amount of energy in a closed system remains constant over time.
In living systems, such as ecosystems, energy changes form as it moves through the food chain, but the total amount of energy remains the same. The main source of energy in most ecosystems is light energy from the Sun, which is captured by producers (plants and algae) during photosynthesis. This process converts light energy into chemical energy stored within the producers. When consumers (organisms at higher levels of the food chain) consume these producers, they gain this stored chemical energy, some of which is then converted into mechanical energy or other forms that power life activities.
As consumers transfer energy to higher levels of the food chain, most of it is "lost" to the environment as thermal energy or heat. However, this energy is not truly lost from the system, only from the food chain. Thus, while the proportion of chemical energy decreases at each stage of the food chain, the total amount of energy in the ecosystem remains constant, in accordance with the law of conservation of energy.
The law of conservation of energy also applies to the human body. For example, the chemical energy from food is converted into thermal energy when broken down by the body, keeping us warm. This illustrates how energy is neither created nor destroyed but only transformed from one form to another, as dictated by the law of conservation of energy.
Demand for Gasoline: Law or Exception?
You may want to see also
Frequently asked questions
The law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another.
Humans need to consume food to get energy as it cannot be created or destroyed.
Plants need energy in the form of sunlight to survive. They convert sunlight into chemical energy through photosynthesis.
During biological processes such as digestion and cellular respiration, some energy is lost as heat.
The energy remains constant.







