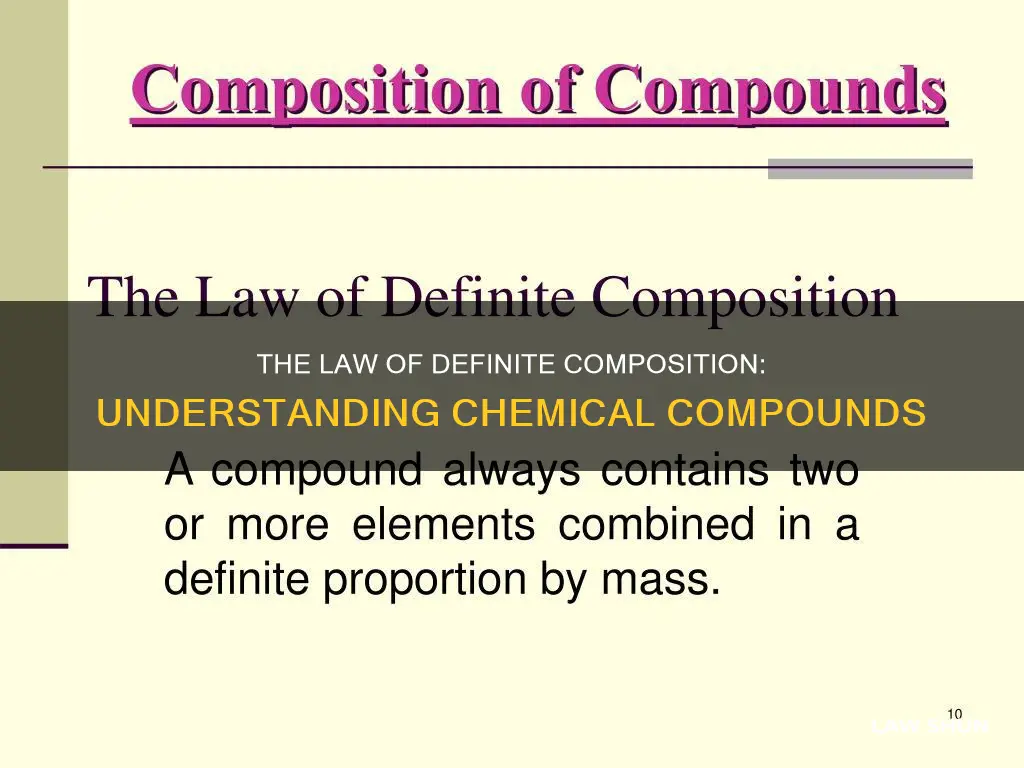
The law of definite composition, also known as Proust's law or the law of constant composition, is a fundamental concept in chemistry. It states that a chemical compound consistently maintains a fixed ratio of its constituent elements by mass, regardless of its source or method of preparation. This principle was first observed by French chemist Joseph Proust in the late 18th century and has since been integral to the development of atomic theory and modern chemistry.
| Characteristics | Values |
|---|---|
| Field | Chemistry |
| Other names | Proust's Law, Law of Constant Composition |
| What it states | That a given chemical compound always contains its component elements in a fixed ratio (by mass) |
| Dependence on source or method of preparation | None |
| Notable examples | Oxygen makes up about 8/9 of the mass of any sample of pure water, while hydrogen makes up the remaining 1/9 of the mass |
| Basis of | Stoichiometry, along with the law of multiple proportions |
| Exceptions | Non-stoichiometric compounds, which are usually metals |
What You'll Learn
- The composition of a compound is fixed, regardless of its source or method of preparation
- The law applies to pure water, which is made up of 8/9 oxygen and 1/9 hydrogen
- The law was first observed by French chemist Joseph Proust in 1794
- The law contributed to John Dalton's atomic theory, which was promoted from 1805
- The law does not apply to non-stoichiometric compounds, which are often metals

The composition of a compound is fixed, regardless of its source or method of preparation
The law of definite composition, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation. This means that the composition of a compound is fixed, regardless of its source or how it was prepared.
For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9. The mass ratio of these two elements in water is always the same, no matter where the sample came from or how it was produced. This law was first observed by French chemist Joseph Proust in 1794, and later formulated by English chemist John Dalton in his chemical atomic theory in 1808.
The law of definite composition is an important concept in chemistry, forming the basis of stoichiometry, along with the law of multiple proportions. It contributed to Dalton's atomic theory, which explained matter as consisting of discrete atoms, with one type of atom for each element, and compounds made of combinations of different types of atoms in fixed proportions. This theory helped to establish the modern understanding of chemical compounds and distinguish them from mixtures.
It is worth noting that there are exceptions to the law of definite composition, known as non-stoichiometric compounds, which are typically metals. These compounds can have varying elemental compositions from sample to sample. However, for the vast majority of chemical compounds, the law of definite composition holds true, providing a fundamental principle in chemistry.
California Labor Laws: Rules for Businesses with Five Employees
You may want to see also

The law applies to pure water, which is made up of 8/9 oxygen and 1/9 hydrogen
The law of definite composition, also known as Proust's law or the law of constant composition, is a chemistry concept. It states that a given chemical compound will always contain its component elements in a fixed ratio by mass, regardless of its source or method of preparation.
This law applies to pure water, which is made up of 8/9 oxygen and 1/9 hydrogen by mass. In other words, any sample of pure water will always contain about 88.81% oxygen and 11.19% hydrogen. This ratio remains constant, no matter where the water sample comes from or how it was prepared.
The law of definite composition was first proposed by Joseph Proust in 1797. At the time, the concept of a chemical compound was not yet fully developed, and Proust's idea was controversial. His fellow Frenchman, Claude Louis Berthollet, disagreed, arguing that elements could combine in any proportion. However, Proust's law eventually contributed to the development of atomic theory, which explained that matter consists of discrete atoms, with compounds formed by combining different types of atoms in fixed proportions.
Today, we understand that water (H2O) is a compound made up of two hydrogen atoms and one oxygen atom. This means that, in terms of mass, oxygen accounts for approximately 88.81% of water, while hydrogen makes up the remaining 11.19%. This ratio is always the same for pure water, regardless of its origin or the methods used to obtain it.
It is important to note that while the law of definite composition holds true for pure compounds like water, it does not apply universally. There are exceptions, such as non-stoichiometric compounds, where the elemental composition can vary from sample to sample.
Applying Ampere's Law: A Practical Guide
You may want to see also

The law was first observed by French chemist Joseph Proust in 1794
The law of definite composition, also known as Proust's law or the law of constant composition, was first observed by French chemist Joseph Proust in the late 18th century. Proust's law states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation.
Proust was born in Angers, France, in 1754 and initially trained as an apothecary, like his father. After relocating to Paris, he studied chemistry with Hilaire-Martin Rouelle and was appointed a pharmacist at the Salpêtrière Hospital in 1776. However, he soon left this position to pursue a career in teaching chemistry, first in Spain and then back in Paris. Proust's interest in aerostatic experiments led him to a balloon ascent with Jean-François Pilâtre de Rozier in 1784. He later returned to Spain, where he continued to teach chemistry and conduct research.
Proust's formulation of the law of definite composition was the result of his experimental work on the composition of various substances, particularly the oxides of iron. He found that chemical substances only truly combine to form a limited number of compounds, and each of these compounds is characterised by elements that combine in fixed proportions by weight. Proust's work on inorganic binary compounds, such as metallic oxides, sulfides, and sulfates, revealed that most metals formed two distinct oxides at constant proportions, which he termed the minimum and maximum.
Proust's law was initially controversial and opposed by other chemists, including his fellow Frenchman Claude Louis Berthollet, who argued that elements could combine in any proportion. However, Proust's findings were supported by Scottish chemist Thomas Thomson, and later by English chemist John Dalton, who incorporated the law into his chemical atomic theory in 1808.
Gas Laws: Everyday Applications and Their Importance
You may want to see also

The law contributed to John Dalton's atomic theory, which was promoted from 1805
The law of definite composition, also known as the law of definite proportions, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation. For example, any sample of pure water contains about 88.8-89% oxygen and 11.19-11% hydrogen by mass, with the mass of the two elements always in the same ratio.
The law of definite proportions was given by Joseph Proust in 1797 and contributed to John Dalton's atomic theory, which was promoted from 1803 or 1805 onwards. Dalton's atomic theory was based on the following assumptions:
- Matter is made up of atoms that are indivisible and indestructible.
- All atoms of an element are identical, and different elements have different types of atoms.
- Atoms of different elements have different weights and chemical properties.
- Atoms of different elements combine in simple whole numbers to form compounds, or 'compound atoms' (molecules).
- Atoms cannot be created or destroyed. When a compound decomposes, the atoms are recovered unchanged.
Dalton's theory was influenced by the work of Joseph-Louis Proust and Claude-Louis Berthollet, who were in disagreement over whether elements could combine in any proportion. Dalton took the fixed proportions for granted and disregarded the controversy, focusing instead on determining the relative masses of each different kind of atom.
Dalton's atomic theory was influential in the development of chemistry, particularly in the field of organic chemistry, and earned him the nickname "father of chemistry".
Charles' Law: Breathe Easy at Depth
You may want to see also

The law does not apply to non-stoichiometric compounds, which are often metals
The law of definite composition, also known as Proust's Law, states that a chemical compound always contains its constituent elements in a fixed ratio by mass. This means that the composition of a compound does not depend on its source or method of preparation. For example, in any sample of pure water, oxygen makes up about 8/9 of the mass, while hydrogen makes up the remaining 1/9.
However, this law does not apply to non-stoichiometric compounds, which are often metals. Non-stoichiometric compounds are those whose elemental composition can vary from sample to sample. In other words, they do not follow the law of constant composition, which states that all samples of a given chemical compound have the same elemental composition by mass.
An example of a non-stoichiometric compound is the iron oxide wüstite, which can contain between 0.83 and 0.95 iron atoms for every oxygen atom. This means that the mass percentage of oxygen in wüstite can vary between 23% and 25%. The ideal formula for wüstite is FeO, but due to crystallographic vacancies, it is closer to Fe0.95O. Proust's measurements were not precise enough to detect such variations.
Another example of a non-stoichiometric compound is titanium oxide (TiO2). The mass percentage of oxygen in titanium oxide can vary depending on the sample. This is because the titanium atom can form multiple stable bonds with oxygen atoms, resulting in different stoichiometric ratios.
In summary, while the law of definite composition is a useful concept in chemistry, it is important to recognize its limitations and understand that it does not apply to all types of compounds, especially non-stoichiometric compounds, which are often metals.
Whistleblower Law: Contractor Rights in Delaware
You may want to see also
Frequently asked questions
The law of definite composition, also known as Proust's law or the law of constant composition, states that a given chemical compound always contains its component elements in a fixed ratio (by mass) and does not depend on its source or method of preparation.
An example of the law of definite composition is water. Any sample of pure water contains about 8/9 of oxygen by mass and 1/9 of hydrogen by mass.
The law of definite composition was first observed by French chemist Joseph Proust in 1794, and later conclusively proven in 1797.
The law of definite composition contributed to the atomic theory that John Dalton promoted beginning in 1805, which explained matter as consisting of discrete atoms, with one type of atom per element, and compounds made of different combinations of atoms in fixed proportions.
While the law of definite composition is an important foundation of modern chemistry, it is not universally true. There are non-stoichiometric compounds, usually metals, whose elemental composition can vary from sample to sample.