Altitude sickness is a condition that occurs when the body fails to adjust to lower oxygen availability at higher altitudes. As altitude increases, the pressure of gases in the body decreases, and various complications can arise. Several gas laws, including Boyle's Law, Dalton's Law, Henry's Law, and Graham's Law, help explain the effects of altitude on the human body and the mechanisms behind altitude sickness. These laws describe the relationships between pressure, volume, and temperature of gases and provide valuable insights into the physiological changes that occur with changes in altitude. Understanding these laws is crucial for preventing and managing altitude sickness, especially in aviation and diving contexts.
| Characteristics | Values |
|---|---|
| Law | Boyle's Law |
| Description | The pressure of a gas is inversely proportional to its volume at a constant temperature. |
| Application to Altitude Sickness | As altitude increases, the volume of gas inside a closed space is subject to expansion. |
| Law | Charles's Law |
| Description | The volume of a gas is directly proportional to its temperature. |
| Application to Altitude Sickness | N/A |
| Law | Dalton's Law |
| Description | The total pressure of a gas mixture is based on the pressures of each component gas. |
| Application to Altitude Sickness | The partial pressure of oxygen is essential, not the percentage of it in the gas we are breathing. |
| Law | Henry's Law |
| Description | The weight of a gas dissolved in a liquid is directly proportional to the weight of the gas above the liquid. |
| Application to Altitude Sickness | Decompression sickness is a direct result of the principles described in Henry's Law. |
What You'll Learn
- Boyle's Law: Pressure and volume are inversely proportional at a constant temperature
- Charles's Law: Volume of a gas is directly proportional to its temperature
- Dalton's Law: Total pressure of a gas mixture is the sum of the partial pressures of its gases
- Henry's Law: Gas solubility in a liquid is directly proportional to the gas's pressure
- Gay-Lussac's Law: Pressure of a gas is directly proportional to its temperature

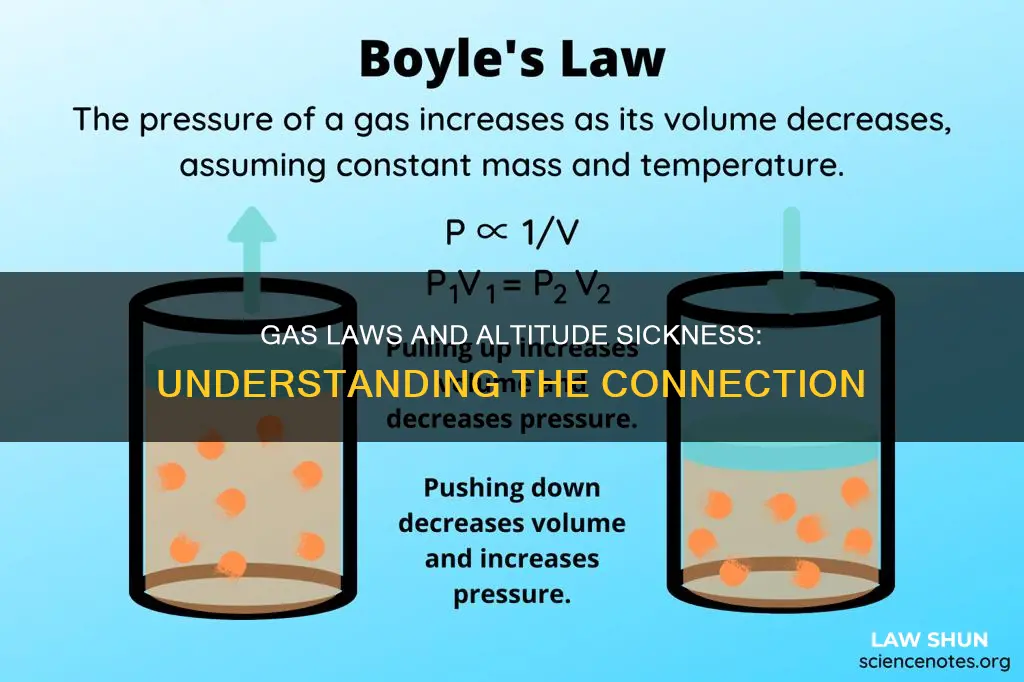
Boyle's Law: Pressure and volume are inversely proportional at a constant temperature
Altitude sickness is a group of medical conditions that occur when an individual moves to a higher altitude too quickly, and the body struggles to adjust to the reduced oxygen availability. The higher the altitude, the thinner the atmosphere, and therefore, the same volume of air results in less oxygen intake.
Boyle's Law, also known as the Boyle-Mariotte Law, is highly relevant to understanding the effects of altitude on the human body. Formulated by Robert Boyle in 1662, it states that at a constant temperature, pressure and volume are inversely proportional to each other. In other words, as one increases, the other decreases.
This law is of utmost importance in understanding the impact of changing atmospheric pressure on the human body when ascending or descending in an aircraft. As an aircraft gains altitude, the volume of gas inside the body expands due to the decrease in external atmospheric pressure. This expansion can lead to various medical complications, such as tension pneumothorax, pneumocephalus, and barosinusitis. Additionally, the law explains the mechanism of air exchange between the atmosphere and the lungs. As the chest cavity expands, the pressure within decreases, causing air to rush in until pressure equalizes.
Furthermore, Boyle's Law has implications for medical equipment and procedures. For instance, it explains the use of saline in the cuff of an endotracheal tube during hyperbaric therapy to prevent air leaks due to volume reduction at increased pressure. It also highlights the need to "burp" colostomy bags as altitude increases to release the expanding gas from stomach contents.
In summary, Boyle's Law, which states that pressure and volume are inversely proportional at a constant temperature, is a critical concept in understanding the effects of altitude on the human body and medical equipment. Its principles help explain various physiological changes and guide safety measures for individuals exposed to changing atmospheric pressures, especially in aircraft or at high altitudes.
Steam Distillation: Understanding Dalton's Law Application
You may want to see also

Charles's Law: Volume of a gas is directly proportional to its temperature
Altitude sickness occurs when the body struggles to adjust to the lower availability of oxygen in the atmosphere at higher altitudes. The air at higher altitudes is often referred to as "thin", but this does not mean that the composition of the air changes. Instead, what changes is the number of oxygen molecules per unit volume of air. This is because the pressure in the atmosphere decreases as altitude increases, and the number of oxygen molecules per unit volume is directly affected by pressure.
The lower temperature at higher altitudes also affects the gases, though not as significantly as the change in pressure. The temperature decreases by approximately 2°C for every additional 1,000 feet of altitude.
Charles's Law states that the volume of a gas is directly proportional to its temperature. This means that if the temperature of a gas increases, the volume of the gas will also increase, and vice versa. This law applies when the pressure on a sample of dry gas is held constant.
Charles's Law can be used to understand the action of a gas thermometer, where the change in volume of a gas is used to display the change in temperature. It can also be observed by placing a balloon filled with gas into a freezer and watching the volume of the balloon decrease.
Charles's Law can be applied to the human body when we breathe in gases. As gases are inspired and warmed from room temperature to body temperature, their volume will increase. For example, an adult breathes in a tidal volume of 500 ml of air at room temperature, which will increase to a volume of 530 ml as it warms up to body temperature.
Charles's Law can also be used to calculate the amount of nitrous oxide remaining in a gas cylinder. The cylinder will contain a mixture of gas and liquid at room temperature, and as the nitrous oxide is removed, the liquid will boil and the gas will expand. By weighing the cylinder, we can calculate the amount of nitrous oxide remaining.
Criminal History: Privacy Laws in Job Applications
You may want to see also

Dalton's Law: Total pressure of a gas mixture is the sum of the partial pressures of its gases
Altitude sickness is caused by a decrease in pressure and temperature, which results in lower oxygen availability as you ascend. This is because the number of oxygen molecules per unit volume of air decreases with altitude, even though the overall composition of gases remains the same.
Now, Dalton's Law states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases in the mixture. This is expressed as:
\[P_{total} = P_A + P_B + ...\]
Where:
- \(P_{total}\) is the total pressure of the gas mixture
- \(P_A\), \(P_B\), etc., are the partial pressures of each individual gas in the mixture
This law is based on the kinetic theory of gases, which states that gases will fill up the container they are in without interacting with each other. Therefore, the pressure of a gas is determined by its collisions with the container, not with other gas molecules. This means that each gas exerts its own pressure independently of the others, and these pressures can simply be added together to find the total pressure.
Dalton's Law can be applied to calculate the partial pressure of oxygen at any altitude, which is critical in understanding the onset and severity of hypoxia, a dangerous condition that can occur at high altitudes. For example, at sea level, the partial pressure of oxygen is 160 mm Hg (21% of 760 mm Hg total pressure). As you ascend, the total pressure decreases, leading to a lower partial pressure of oxygen.
It's important to note that Dalton's Law assumes constant temperature and volume. In reality, temperature and volume can also change with altitude, adding further complexity to the behaviour of gases at high altitudes.
Equal Justice Under Law: How It Applies Today
You may want to see also

Henry's Law: Gas solubility in a liquid is directly proportional to the gas's pressure
Altitude sickness is a condition that occurs when the body doesn't have enough time to adjust to the lower oxygen availability at higher altitudes. As altitude increases, atmospheric pressure decreases, and the number of oxygen molecules per unit volume of air decreases as well. This results in less oxygen being available with each breath, even though the percentage composition of oxygen in the air remains the same.
Henry's Law, named after English physician William Henry, describes the relationship between the partial pressure of gases in a gaseous mixture (like the atmosphere) and the amount of that gas that can dissolve in a solution (like blood). The law states that the amount of dissolved gas in a liquid is directly proportional to the partial pressure of that gas. In other words, if the pressure of a gas above a liquid increases, the amount of gas that can dissolve in that liquid also increases proportionally. Conversely, if the gas pressure decreases, the amount of dissolved gas in the solution drops.
At sea level, the partial pressure of gases in the air and in our blood are roughly equal, allowing for equilibrium. However, as altitude increases, the partial pressure of gases in the atmosphere decreases, leading to a disruption in this balance. According to Henry's Law, this decrease in partial pressure results in a decrease in the amount of gas that can remain dissolved in our blood. This can cause certain gases, like nitrogen, to "bubble out" of the bloodstream, leading to various forms of decompression sickness.
The effects of Henry's Law are particularly relevant in aerospace medicine, where it helps explain the occurrence of decompression sickness in pilots, aircrew, and passengers. Cabin pressurization and controlled rates of ascent are used to mitigate the effects of Henry's Law and reduce the risk of decompression sickness during flights.
Additionally, Henry's Law can be applied to understand the impact of altitude on gases in closed cavities within the body. As altitude increases and ambient pressure decreases, the volume of gases in these closed spaces can expand, potentially leading to issues such as pneumothorax.
The Applicability of Gas Laws to Liquids and Solutions
You may want to see also

Gay-Lussac's Law: Pressure of a gas is directly proportional to its temperature
Gay-Lussac's Law, also known as the Third Gas Law, states that the pressure exerted by a gas is directly proportional to its temperature when the volume is held constant. This law was formulated by French chemist Joseph Gay-Lussac in 1808 and is particularly relevant when considering the behaviour of gases in closed systems.
Mathematically, Gay-Lussac's Law can be expressed as:
P ∝ T
Where:
- P is the pressure exerted by the gas
- T is the absolute temperature of the gas
The formula for Gay-Lussac's Law is:
P1/T1 = P2/T2 = k
Where:
- P1 and T1 are the initial pressure and temperature
- P2 and T2 are the final pressure and temperature
- K is a constant
This law implies that the ratio of initial pressure to temperature is equal to the ratio of final pressure to temperature for a fixed mass of gas kept at a constant volume.
Gay-Lussac's Law is similar to Charles' Law, with the key difference being the type of container used. In Gay-Lussac's Law experiments, the container is rigid, while in Charles' Law experiments, the container is flexible.
Gay-Lussac's Law has various practical applications. For example, it explains why gauges measuring the pressure inside propane tanks register higher pressure on hot days than on cool days. It also explains why pressurised aerosol cans have warning labels instructing users to keep them away from fire and store them in a cool place. When heated, the increase in pressure exerted by the gases inside the can may lead to an explosion.
Gay-Lussac's Law is relevant to understanding altitude sickness. As altitude increases, air pressure decreases, and the human body may struggle to adjust to the reduced oxygen availability. Gay-Lussac's Law helps explain this phenomenon by describing the relationship between pressure and temperature in gases.
Scientific Laws Governing Systems: Unlocking the Trifecta
You may want to see also
Frequently asked questions
Altitude sickness is a term for medical conditions that occur when you move to a higher altitude too quickly, and your body doesn't have time to adjust to the lower oxygen availability.
Symptoms of altitude sickness include headache, nausea and vomiting, fatigue, malaise, dizziness, and vision changes.
Boyle's Law states that the pressure of a gas is inversely proportional to its volume at a constant temperature. As altitude increases, the volume of gas inside a closed space expands due to decreased external pressure. This can have implications for conditions such as pneumothorax, pneumocephalus, and barosinusitis.
Dalton's Law states that the total pressure of a gas mixture is equal to the sum of the partial pressures of its constituent gases. This law is important in understanding the partial pressure of oxygen at higher altitudes, which can be crucial in preventing altitude sickness.
Henry's Law states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of that gas. This law explains decompression sickness, which can occur when a diver ascends too quickly from a significant depth, leading to the formation of nitrogen bubbles in the veins and tissues.







