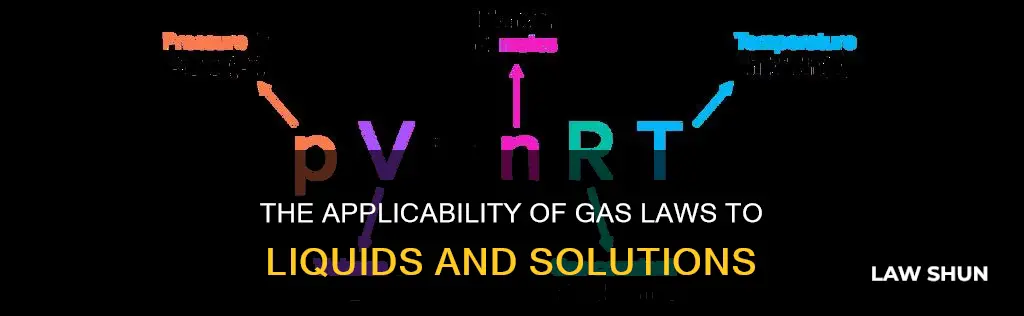
The ideal gas law applies only to ideal gases. Liquids have a constant volume, and the ideal gas law is expressed by simpler gas laws such as Boyle's law, Charles's law, Avogadro's law, and Amonton's law, which deal with the pressure, volume, and temperature of a gas. The ideal gas law assumes that gas particles do not occupy any space and are in constant, random, and straight-line motion, which is not applicable to liquids. Therefore, the ideal gas law does not apply to liquids.
| Characteristics | Values |
|---|---|
| Applicability | Only applies to ideal gases |
| Volume | Liquids have a constant volume |
| Intermolecular attraction | Present in liquids |
| Density | Liquids have higher densities |
What You'll Learn
- Liquids have a constant volume, unlike the variable volume of gases
- Intermolecular attraction provides the characteristic shape and properties of liquids
- Liquids have a higher density than gases
- Liquids do not have a concentration and so cannot be used in the ideal gas equation
- The ideal gas law is derived from a model that assumes particles occupy no volume

Liquids have a constant volume, unlike the variable volume of gases
The Ideal Gas Law cannot be applied to liquids. This is because the volume of a liquid is constant, whereas the volume of a gas is variable. Liquids have a nearly constant volume independent of pressure. They are nearly incompressible, meaning that they occupy nearly a constant volume over a wide range of pressures.
Liquids conform to the shape of their container but maintain a fairly constant density. They do not generally expand to fill the available space in a container but form their own surface. The molecules in a liquid are very close together, barely further apart than in solids, and are bound firmly but not rigidly. This means that liquids do not easily compress, so their volume is fixed.
Gases, on the other hand, are very different by nature. The particles in gases are very small, so they do not occupy any space. They have constant, random, and straight-line motion. Gas particles travel randomly, and elastic collisions occur randomly.
The Ideal Gas Law deals with the pressure, volume, and temperature of a gas in the limit of low pressures and high temperatures. It is expressed by simpler gas laws such as Boyle's Law, Charles's Law, Avogadro's Law, and Amonton's Law.
Antitrust Laws: Global Reach and Overseas Application
You may want to see also

Intermolecular attraction provides the characteristic shape and properties of liquids
The ideal gas law applies only to gases, and not to solids or liquids. This is because gases are fundamentally different from solids and liquids, which exhibit intermolecular attractions that provide their characteristic shape and properties. Liquids, for instance, have a constant volume, which contradicts the ideal gas law.
Intermolecular attraction, or IMF, is the term used to describe the attractive forces between the particles of a substance, be they atoms, molecules, or ions. These forces are responsible for the characteristic shape and properties of liquids. The strength of IMFs varies depending on the substance in question, and they can be influenced by factors such as the size and weight of the atoms or molecules involved, as well as the shape of the molecules.
The types of IMFs that can occur between atoms or molecules in condensed phases include dispersion forces, dipole-dipole attractions, and hydrogen bonding. Dispersion forces are relatively weak and occur when the electrons of an atom or molecule are distributed asymmetrically, creating a temporary dipole that can distort the electrons of a neighbouring atom or molecule, resulting in an induced dipole. Larger and heavier atoms and molecules tend to exhibit stronger dispersion forces.
Dipole-dipole attractions occur between polar molecules, where there is a partial positive charge on one side and a partial negative charge on the other. The attraction is between the positive end of one molecule and the negative end of another. This type of IMF is stronger than dispersion forces and can cause molecules to "stick together" to form a liquid, as seen with hydrogen chloride (HCl) molecules.
Hydrogen bonding is a particularly strong form of dipole-dipole attraction that occurs when a molecule contains a hydrogen atom bonded to a highly electronegative element such as fluorine, oxygen, or nitrogen. Hydrogen bonds are much weaker than covalent bonds but are generally stronger than other dipole-dipole attractions and dispersion forces. They have a significant impact on the properties of condensed phases, such as liquids.
The strength of IMFs in liquids can also be influenced by factors such as branching in isomeric alkanes, which can increase boiling points by lowering the intermolecular van der Waals force of attraction. Additionally, the boiling point of a liquid is inversely proportional to the strength of its intermolecular forces of attraction—the weaker the IMFs, the higher the boiling point.
Fair Lending Laws: Commercial Loans and Their Exemptions
You may want to see also

Liquids have a higher density than gases
The Ideal Gas Law cannot be applied to liquids. This is because the law deals with the pressure, volume, and temperature of a gas, and the behaviour of an ideal gas is interpretable. The particles in a gas are very small, and they do not occupy any space.
Liquids, on the other hand, have a constant volume. The particles in liquids can move around more freely, but they slide over each other with some gaps between them. This means that liquids typically have a lower density than solids but a higher density than gases.
In gases, the particles are far apart, moving quickly in random directions with a lot of empty space between them. So they have the lowest density of all.
Understanding Labor Law Protections for H-1B Visa Holders
You may want to see also

Liquids do not have a concentration and so cannot be used in the ideal gas equation
The Ideal Gas Law applies only to ideal gases. Liquids aren’t gases, and therefore the Ideal Gas Law does not apply to them. Liquids have a constant volume, whereas the Ideal Gas Law requires volume to be a variable.
The Ideal Gas Law, expressed as PV = nRT, is used to understand the behaviour of gases under certain, specific conditions. It is specifically for gases as it includes moles and volume (which can be manipulated to give concentration n/v). Liquids and solids do not have a "concentration", and so cannot be used in the Ideal Gas Law.
The Ideal Gas Law is derived from a model (the ideal gas), and like every other model, it applies where its underlying assumptions are good approximations of reality. For example, the ideal gas assumes point particles, non-interacting molecules, and random motion. Liquids, by their nature, do not meet these criteria.
Liquids are also denser than gases, and have intermolecular attractions which give them their characteristic shape and properties. This means that gas laws are not applicable to liquids.
Music Copyright: Public Performance and Amateur Musicians
You may want to see also

The ideal gas law is derived from a model that assumes particles occupy no volume
The ideal gas law applies to gases only, and not to solids or liquids. This is because gases are fundamentally different from solids and liquids. The ideal gas law is derived from a model that assumes particles occupy no volume. This assumption does not hold for liquids, as the particles in liquids are closer together and are quite sensitive to the forces between them.
The ideal gas law is expressed as:
> PV = nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the amount of substance of gas (also known as the number of moles)
- R is the ideal or universal gas constant
- T is the absolute temperature of the gas
The ideal gas law can also be written as:
> PV = NkT
Where:
- P is the pressure of the gas
- V is the volume it occupies
- N is the number of atoms and molecules in the gas
- K is the Boltzmann constant
- T is its absolute temperature
The ideal gas law is derived from basic principles but was originally deduced from experimental measurements of Charles' Law and Boyle's Law. In the ideal gas model, the volume occupied by its atoms and molecules is a negligible fraction of V. The ideal gas law describes the behaviour of real gases under most conditions.
The ideal gas law applies only to gases as the behaviour of an ideal gas is interpretable. The particles in a gas are very small, so they do not occupy any space. Gases are also different from solids and liquids by nature. The intermolecular attraction is present in solids and liquids and gives them their characteristic shape and properties. Therefore, gas laws do not apply to solids and liquids.
GDPR Laws: Implications for WHO and Global Health
You may want to see also
Frequently asked questions
The ideal gas law applies only to ideal gases. Liquids are not gases, and they have different properties, such as having a constant volume, unlike gases.
Gases are made up of particles that are in constant, random, and straight-line motion. These particles are very small and do not occupy any space. Liquids, on the other hand, have higher densities and intermolecular attractions that give them their characteristic shape and properties.
While the ideal gas law is specifically derived for gases, it can be applied to describe the exact entropy of a dilute solution in a dense liquid. This is because the entropy of a dilute solution in a dense liquid is the same as that of a dilute gas, as they have the same number of possible positions for the solute particles.