The law of thermal expansion, which states that the volume of a material changes when its temperature changes, applies to ocean water. As the Earth's temperature rises due to the accumulation of heat-trapping greenhouse gases, the oceans absorb over 90% of this heat. As a result, ocean temperatures rise, causing the water to expand and contributing to an increase in global sea levels. This phenomenon, known as thermal expansion, has been observed through measurements taken by ships, satellites, and drifting sensors, as well as subsurface observations. The impact of thermal expansion on sea level rise is a significant concern in the context of climate change, with rising sea levels posing risks to coastal areas and communities.
| Characteristics | Values |
|---|---|
| What is thermal expansion? | When an object is heated, its atoms vibrate faster and spread out, causing the object to expand. |
| How does it work with water? | When water gets warmer, the volume of the water increases. |
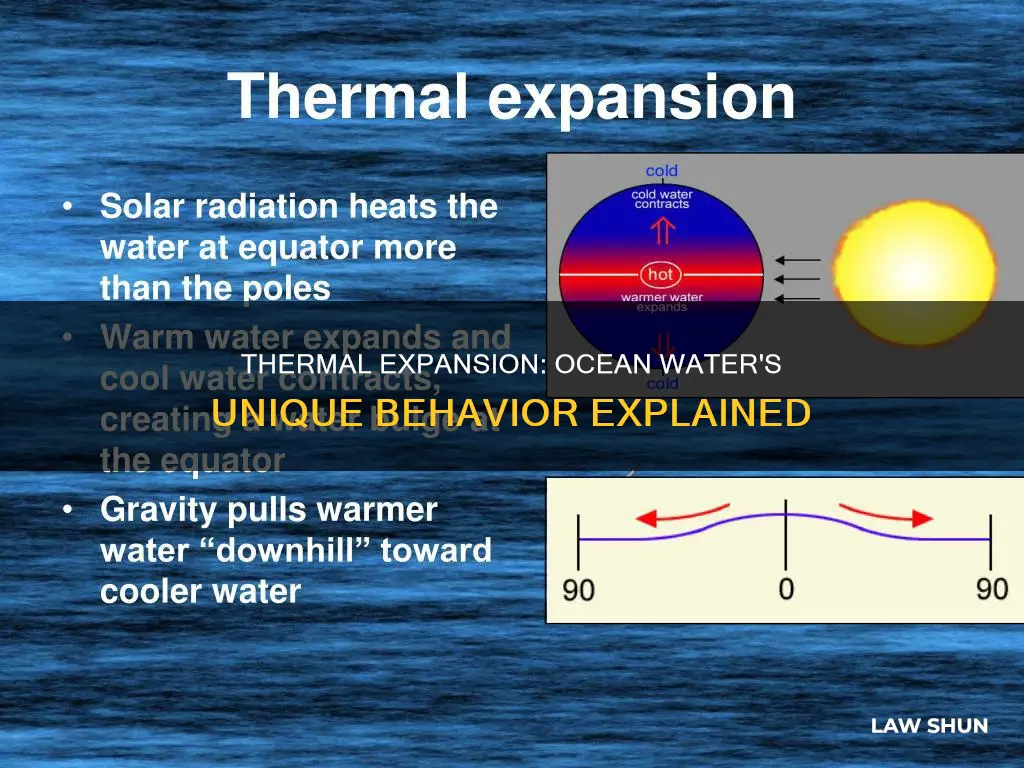
| How does this affect the ocean? | The ocean's surface readily takes in heat from the air, causing the molecules at the surface to heat up and expand. |
| How much does it contribute to rising sea levels? | About half of the measured global sea level rise on Earth is from warming waters and thermal expansion. |
| How does depth impact thermal expansion? | At greater depths and higher pressures, water expands even more, which means that for the same heat input, ocean water will expand more at deeper depths than water at the surface. |
What You'll Learn
- How does thermal expansion of ocean water affect sea levels?
- How does the coefficient of thermal expansion differ for saltwater and freshwater?
- What is the impact of thermal expansion on coastal flooding and erosion?
- How does thermal expansion of ocean water contribute to climate change?
- How can the thermal expansion of ocean water be demonstrated through a model?

How does thermal expansion of ocean water affect sea levels?
The law of thermal expansion applies to ocean water, and this has a significant impact on sea levels. As the Earth's temperature rises, the oceans absorb over 90% of this heat, leading to a rise in ocean temperatures and subsequently, an increase in water volume. This is due to the molecules in the water becoming more "excited" and taking up more space. This process is known as thermal expansion and contributes to an increase in global sea levels.
The warming of ocean water and its subsequent expansion is a primary concern in the context of climate change. The coefficient of thermal expansion, which determines the change in volume relative to temperature change, is unique to each material. In the case of ocean water, this coefficient is sensitive to ambient temperature and pressure. As a result, water at greater depths and higher pressures will expand more for a given increase in temperature, leading to an even greater impact on sea levels.
The impact of thermal expansion on sea levels is not uniform across the globe. Ocean currents distribute heat unevenly, resulting in larger sea level rises in certain regions. Additionally, some land surfaces, such as delta regions, are more vulnerable to rising sea levels due to gradual subsidence.
The rate of thermal expansion is also influenced by the amount of greenhouse gas emissions. As emissions increase, the rate of thermal expansion accelerates, contributing to a faster rise in sea levels. This acceleration in sea level rise has already led to more frequent coastal flooding and erosion in many regions.
Furthermore, the impact of thermal expansion on sea levels is compounded by the melting of glaciers and ice caps, which act as a cooling mechanism for the deep sea. With the loss of this cooling mechanism, the ocean's ability to overturn is impaired, leading to more rapid hyper-warming of its waters and further increases in sea levels.
In summary, the thermal expansion of ocean water significantly affects sea levels. This process is influenced by factors such as water depth, pressure, and the rate of temperature change. The consequences of thermal expansion are already being felt in the form of increased coastal flooding and erosion, and the impact is expected to grow unless measures are taken to mitigate climate change.
Intimidation and the Law: Where Does It Apply?
You may want to see also

How does the coefficient of thermal expansion differ for saltwater and freshwater?
The coefficient of thermal expansion is a unique property of materials that determines how much their volume will change when heated. It is not a universal constant and varies with the type of material, ambient temperature, and pressure.
When it comes to saltwater and freshwater, the coefficient of thermal expansion differs due to the presence of salt in saltwater. Salt dissociates into ions, such as sodium (Na+) and chloride (Cl-) ions, which occupy the space between water molecules and reduce the number of hydrogen bonds between them. This results in water molecules being less tightly bonded, leading to a greater change in volume when heated compared to distilled water. In other words, saltwater has a higher coefficient of volumetric expansion than freshwater.
The coefficient of thermal expansion for water is given by the equation:
ΔV = βV0ΔT
Where ΔV is the change in volume, β is the coefficient of thermal expansion, V0 is the original volume, and ΔT is the change in temperature in degrees Celsius. The value of β for water is not constant and changes with temperature. For example, at 10°C, β is approximately 0.88 x 10^-4/°C, while at 20°C, it increases to 2.07 x 10^-4/°C.
For seawater with an average salinity of 3.5%, the coefficient of thermal expansion is about 25% higher compared to pure water. This means that for the same increase in temperature, saltwater will expand more than freshwater. The mutual repulsion of ions in saltwater contributes to this effect.
The difference in the coefficient of thermal expansion between saltwater and freshwater has significant implications for our planet. As the Earth's temperature rises due to the accumulation of heat-trapping greenhouse gases, the oceans absorb more than 90% of this trapped heat. This leads to a rise in ocean temperatures, causing the water to expand due to thermal expansion, which contributes to an increase in global sea levels.
Obscenity Law: Does It Cover Slurs and Hate Speech?
You may want to see also

What is the impact of thermal expansion on coastal flooding and erosion?
As the Earth's temperature increases, the ocean absorbs over 90% of the trapped heat, causing ocean temperatures to rise and seawater to expand. This thermal expansion contributes to an increase in global sea levels. Even small changes in ocean temperature can produce a significant sea level rise effect when considered over time.
The impact of thermal expansion on coastal flooding and erosion is twofold. Firstly, rising sea levels caused by thermal expansion increase the likelihood of coastal flooding. Coastal erosion costs roughly $500 million per year for coastal property loss, including damage to structures and loss of land. Secondly, thermal expansion accelerates with increasing temperatures, leading to an increase in coastal erosion rates. The combination of storm surges and high waves associated with tropical storms creates the most damaging conditions for coastal erosion.
The human response to coastal erosion will be critical in mitigating its impacts. Structural solutions, such as seawalls and groins, may cause more problems than they solve by interfering with natural water currents and preventing sand from shifting along coastlines. Non-structural solutions, such as beach nourishment and wetland restoration, are increasingly favored as they enhance the natural ability of shorelines to absorb and dissipate storm energy.
Lemon Law: Does It Cover Air Conditioners?
You may want to see also

How does thermal expansion of ocean water contribute to climate change?
The law of thermal expansion applies to ocean water, and this has a significant impact on climate change. As the Earth's temperature rises due to the accumulation of heat-trapping greenhouse gases, the oceans absorb over 90% of this heat. This leads to a rise in ocean temperatures, causing the water to expand. The process, known as thermal expansion, contributes to the global sea level rise.
Thermal expansion of ocean water is a critical factor in climate change and has far-reaching consequences. Firstly, it leads to an increase in global sea levels, which poses a significant risk to low-lying coastal areas. These regions are home to almost a billion people, and even slight sea level rises can have devastating impacts. Secondly, higher sea levels can exacerbate the impact of severe storms, leading to more frequent and intense coastal flooding and erosion. This phenomenon is already being observed in many parts of the world, causing significant social and economic disruptions.
Furthermore, the rise in sea levels due to thermal expansion is not uniform. Ocean currents distribute heat unevenly, resulting in varying rates of sea level rise in different regions. Some coastal areas, such as delta regions, are more vulnerable to rising sea levels due to gradual subsidence. This variability in sea level rise has significant implications for coastal communities and infrastructure.
Additionally, the warming of the oceans through thermal expansion has implications for ocean circulation patterns. Warmer water is less dense, which can affect the formation of deep water and the functioning of the global ocean conveyor belt. This, in turn, can have far-reaching consequences for climate patterns and the distribution of heat and nutrients in the oceans.
Moreover, thermal expansion contributes to the positive feedback loop of climate change. As the oceans warm, they become less effective at absorbing CO2, which further accelerates the rate of climate change. This positive feedback mechanism has significant implications for the future of our planet.
In conclusion, the thermal expansion of ocean water is a critical component of climate change. It contributes to rising sea levels, exacerbates the impacts of severe weather events, and influences ocean circulation patterns. Addressing and adapting to the challenges posed by thermal expansion will be essential for mitigating the impacts of climate change on coastal communities and ecosystems.
Independent Assortment: Universal Gene Law?
You may want to see also

How can the thermal expansion of ocean water be demonstrated through a model?
The thermal expansion of ocean water can be demonstrated through a model using a simple setup of everyday items. This model can be built and observed by students to understand how the addition of heat energy causes water to expand, leading to rising sea levels. Here's a step-by-step guide to constructing and using the model:
Materials:
- 1 disposable plastic water bottle, preferably with a thick, sturdy structure and a flip-top lid.
- 1 clear plastic straw.
- Cutting tool (optional).
- Thermometer (optional).
- Low-temperature hot-glue gun, putty, or other malleable sealant.
- Clay or similar material.
- Paper or cloth towels.
- Heat source such as incandescent light bulbs, heat lamps, heating pads, or sunlight.
- Food coloring (optional).
Procedure:
- Prepare the Bottle: Fill the bottle with water to the rim. If desired, add food coloring or tea to make the water more visible.
- Prepare the Straw: Wrap clay or putty around the straw, leaving 2-3 inches (5-8 cm) of straw exposed at both ends. Ensure no gaps are left between the straw and the clay to prevent water leakage.
- Insert the Thermometer (optional): You can either apply a stick-on thermometer to the side of the bottle, facing away from the heat source, or poke a small hole in the clay to insert a thermometer probe. Ensure the thermometer does not interfere with the straw.
- Assemble the Bottle and Straw: Insert the straw into the bottle, with the sealed end reaching about 2-3 inches into the bottle. Seal the top of the bottle using clay, putty, or another sealant to prevent water leakage.
- Prepare to Measure: Place the bottle in a location where you can direct a heat source at it or expose it to direct sunlight. Mark a line on the straw to indicate the initial water level, or "zero-level." Avoid moving or handling the bottle once the line is marked to prevent alterations in measurements.
- Apply Heat: Direct the heat source at the bottle or place it in direct sunlight. Regularly measure and record the water level in millimeters at consistent intervals (every minute or five minutes). If using a thermometer, record the temperature at these intervals as well.
- Observe and Analyze: As heat energy is added, observe and record the changes in the water level. Graph your measurements on paper or using spreadsheet software. Compare the initial and final water levels to determine the extent of thermal expansion.
- Discuss and Conclude: Discuss the relationship between the increase in water temperature and the rise in water level. Explain how this model represents the impact of global temperature rise on ocean water, leading to thermal expansion and subsequent sea-level rise.
This model effectively demonstrates the thermal expansion of ocean water by illustrating how the addition of heat energy causes the water to expand and occupy more space. This expansion contributes to the overall rise in sea levels, which has significant implications for coastal regions and climate change.
Moore's Law and Quantum Computing: A Match?
You may want to see also
Frequently asked questions
Yes, the law of thermal expansion applies to ocean water. As the ocean's temperature rises, the volume of seawater increases, causing sea levels to rise.
As the ocean's surface heats up, the molecules at the surface expand. This is known as thermosteric sea level rise. At greater depths and higher pressures, water expands even more, resulting in higher sea levels.
Thermal expansion is a significant contributor to global sea level rise. Between 1993 and 2010, the thermal expansion of the oceans accounted for approximately 34% of the total sea level rise, at a rate of 1.1 mm per year.