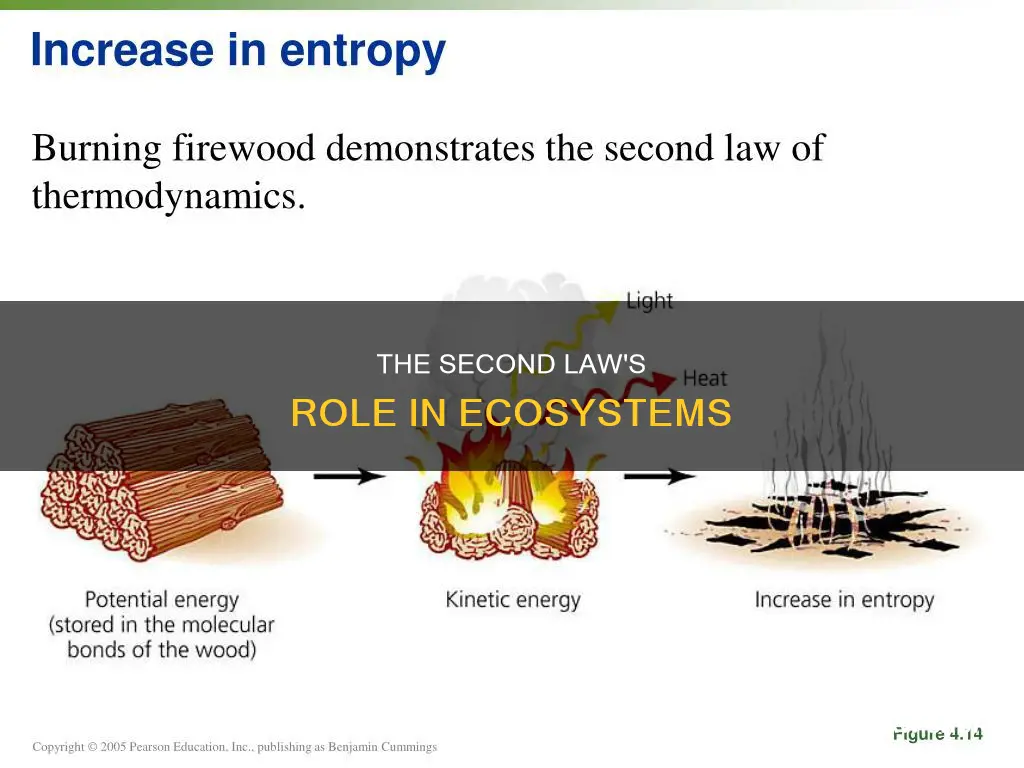
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is, the entropy increases. This law is concerned not with the quantity but with the quality of energy. In an ecosystem, the producers obtain energy from the sun and convert it into organic matter, which increases the entropy. This energy is then transferred to the primary consumers, secondary consumers, and finally to tertiary consumers. Each trophic level receives 10% of energy from the previous trophic level. The rest of the energy is lost as heat, due to the respiration of the organisms, into the environment, resulting in the overall increase in entropy of the system. Therefore, at each trophic level, the entropy increases following the second law of thermodynamics.
| Characteristics | Values |
|---|---|
| --- | --- |
| Energy flow in an ecosystem | The energy flow in an ecosystem follows the second law of thermodynamics. |
| Producers obtain energy from the sun and convert it into organic matter, increasing the entropy. | |
| This energy is transferred to the primary, secondary and tertiary consumers. | |
| Each trophic level receives 10% of energy from the previous trophic level. | |
| The rest of the energy is lost as heat into the environment, resulting in an overall increase in entropy. | |
| At each trophic level, the entropy increases following the second law of thermodynamics. |
What You'll Learn
- The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is the entropy increases
- The energy flow in an ecosystem: Producers obtain energy from the sun and convert it into organic matter, this increases the entropy
- This energy is transferred to the primary consumers, secondary consumers and finally to tertiary consumers. Each trophic level receives 10% of energy from the previous trophic level
- The rest of the energy is lost as heat, due to the respiration of the organisms, into the environment resulting in the overall increase in entropy of the system
- Therefore at each trophic level the entropy increases following the second law of thermodynamics

The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is the entropy increases
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is, the entropy increases. This law is concerned not with the quantity but with the quality of energy. In an ecosystem, the producers obtain energy from the sun and convert it into organic matter, which increases the entropy. This energy is then transferred to the primary, secondary, and tertiary consumers. Each trophic level receives about 10% of the energy from the previous level, with the rest lost as heat due to the respiration of the organisms into the environment, resulting in an overall increase in the entropy of the system.
The second law of thermodynamics can be applied to ecosystems, which are not isolated systems. However, the law still holds true for these systems as they are open systems that can exchange energy and matter with their surroundings. The entropy of an ecosystem tends to increase over time, as energy is constantly being added to the system from external sources, such as the sun. This added energy increases the disorder or randomness of the system, leading to an increase in entropy.
The second law of thermodynamics also implies that the efficiency of energy conversion processes in ecosystems is never 100%. There is always a loss of energy in the form of heat, which contributes to the increase in entropy. This loss of energy can be observed in the food chain, where only about 10% of the energy is passed on to the next trophic level. The remaining 90% is lost as heat through processes such as respiration and metabolic activities.
The second law of thermodynamics also has implications for the stability and sustainability of ecosystems. As the entropy of an ecosystem increases, it moves further away from equilibrium, becoming more disordered and unpredictable. This can lead to a decrease in the ecosystem's stability and resilience, making it more vulnerable to disturbances and external influences.
Overall, the second law of thermodynamics highlights the tendency of ecosystems to move towards a state of higher entropy, or disorder. This increase in entropy is driven by the constant addition of energy from external sources and the loss of energy through processes such as respiration and metabolic activities.
Insider Trading Laws: Private Companies and Legal Boundaries
You may want to see also

The energy flow in an ecosystem: Producers obtain energy from the sun and convert it into organic matter, this increases the entropy
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is, the entropy increases. This law is concerned not with the quantity but with the quality of energy.
In an ecosystem, the producers obtain energy from the sun and convert it into organic matter, which increases the entropy. This energy is then transferred to the primary, secondary, and tertiary consumers, with each trophic level receiving 10% of the energy from the previous level. The rest of the energy is lost as heat due to the respiration of the organisms and is released into the environment, resulting in an overall increase in the entropy of the system. Therefore, at each trophic level, the entropy increases, following the second law of thermodynamics.
The energy flow in an ecosystem can be seen as linear, with energy being passed from the sun to the producers, and then to the consumers. This is in keeping with the first law of thermodynamics, which states that energy can be transferred or transformed but cannot be created or destroyed.
The increase in entropy in an ecosystem can also be viewed from the perspective of exergy, which is the maximum work that can be extracted from a system when it is brought to equilibrium with its surroundings. Exergy has been proposed as an important factor in assessing the integrity of ecosystems and has been used as a goal function in ecological modelling.
The concept of exergy optimization states that biological systems should tend to optimize their exergy (storage). This has been referred to as the ecological law of thermodynamics and is seen as a translation of the concept of "survival of the fittest" into thermodynamics. Exergy optimization can be seen as a strategy for biological systems to maintain their internal energy values at the highest possible level for as long as possible.
Overall, the application of thermodynamics to ecology has provided insights into the functioning and development of ecosystems, offering a scientific foundation for concepts such as sustainability, resilience, and ecosystem health.
Oregon's Concealed Carry Laws: Antique Firearms Included?
You may want to see also

This energy is transferred to the primary consumers, secondary consumers and finally to tertiary consumers. Each trophic level receives 10% of energy from the previous trophic level
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is, the entropy increases. This law is concerned not with the quantity but with the quality of energy. In an ecosystem, the energy flow follows this law. The energy from the sun is converted into organic matter by the producers, which increases the entropy. This energy is then transferred to the primary consumers, secondary consumers, and finally to tertiary consumers. Each trophic level receives 10% of energy from the previous level. The rest of the energy is lost as heat due to the respiration of the organisms, resulting in an overall increase in the entropy of the system.
The unidirectional flow of energy in an ecosystem is governed by the second law of thermodynamics. The energy flow is from the sun to the producers and then to the consumers. The energy flow is shown using food chains, which are then organised into trophic pyramids to more efficiently show the quantity of organisms at each trophic level. The arrows in the food chain indicate the direction of the energy flow, which is always unidirectional.
The energy flow in an ecosystem can also be represented using an energy flow diagram, which is used to show energy and energy transformation visually and quantitatively.
HIPAA Laws: Who Is Bound and Who Is Exempt?
You may want to see also

The rest of the energy is lost as heat, due to the respiration of the organisms, into the environment resulting in the overall increase in entropy of the system
The second law of thermodynamics states that the entropy of an isolated system will always increase over time. This means that the disorder of a system will increase, and the energy available to do work will decrease. This is because heat always flows from hot to cold regions of matter, and heat transfer of energy from hot to cold increases the entropy of the system.
The second law can be applied to the respiration of organisms within an ecosystem. In an ecosystem, producers obtain energy from the sun and convert it into organic matter, increasing the entropy. This energy is then transferred to primary, secondary, and tertiary consumers. Each trophic level receives 10% of the energy from the previous level, with the rest of the energy being lost as heat due to the respiration of the organisms. This results in an overall increase in entropy in the system.
Debt Collection Laws: Do They Apply to Businesses?
You may want to see also

Therefore at each trophic level the entropy increases following the second law of thermodynamics
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is the entropy increases. This law is concerned not with the quantity but with the quality of energy.
In an ecosystem, the producers obtain energy from the sun and convert it into organic matter, this increases the entropy. This energy is then transferred to the primary, secondary, and tertiary consumers. Each trophic level receives 10% of energy from the previous trophic level. The rest of the energy is lost as heat due to the respiration of the organisms, into the environment resulting in the overall increase in entropy of the system. Therefore, at each trophic level, the entropy increases following the second law of thermodynamics.
Murphy's Law: Wisconsin's Exception or Rule?
You may want to see also
Frequently asked questions
The second law of thermodynamics states that there is a natural tendency of any isolated system to move towards a disorderly state, that is the entropy increases. This law is concerned not with the quantity but with the quality of energy.
The second law of thermodynamics applies to an ecosystem in the following ways:
- In an ecosystem, the producers obtain the energy from the sun and convert it into organic matter, this increases the entropy.
- This energy is transferred to the primary consumers, secondary consumers, and finally to tertiary consumers. Each trophic level receives 10% of energy from the previous trophic level. The rest of the energy is lost as heat, due to the respiration of the organisms, into the environment resulting in the overall increase in entropy of the system.
- At each trophic level, the entropy increases following the second law of thermodynamics.
The second law of thermodynamics is significant because it places restrictions on heat engines—devices that transform heat into electrical, mechanical, and chemical energy.







