Boyle's Law, discovered by Anglo-Irish chemist Robert Boyle in 1662, states that the pressure exerted by a gas is inversely proportional to the volume it occupies, as long as the temperature and the quantity of gas remain constant. This law can be applied to understand the effects of altitude on the human body, particularly in relation to pressure and volume changes. As altitude increases, the pressure exerted on gases within closed cavities in the body also increases, leading to volume expansion. This can have significant implications for aviation medicine and mountain climbing, where changes in pressure and oxygen levels at high altitudes can result in health issues such as hypoxia, barotrauma, and high-altitude illnesses.
| Characteristics | Values |
|---|---|
| What is Boyle's Law? | Boyle's law, also referred to as the Boyle–Mariotte law or Mariotte's law, is an empirical gas law that describes the relationship between pressure and volume of a confined gas. |
| Who was it named after? | Robert Boyle |
| When was it published? | 1662 |
| What does it state? | The absolute pressure exerted by a given mass of an ideal gas is inversely proportional to the volume it occupies if the temperature and amount of gas remain unchanged within a closed system. |
| What is the mathematical representation? | P ∝ (1/V) or P.V = k, where k is a constant and is dependent on the temperature. |
| What is the formula? | P = k*(1/V) ⇒ PV = k |
| What is the curve between pressure and volume like? | A straight line is obtained when the pressure exerted by the gas (P) is taken on the Y-axis and the inverse of the volume occupied by the gas (1/V) is taken on the X-axis. |
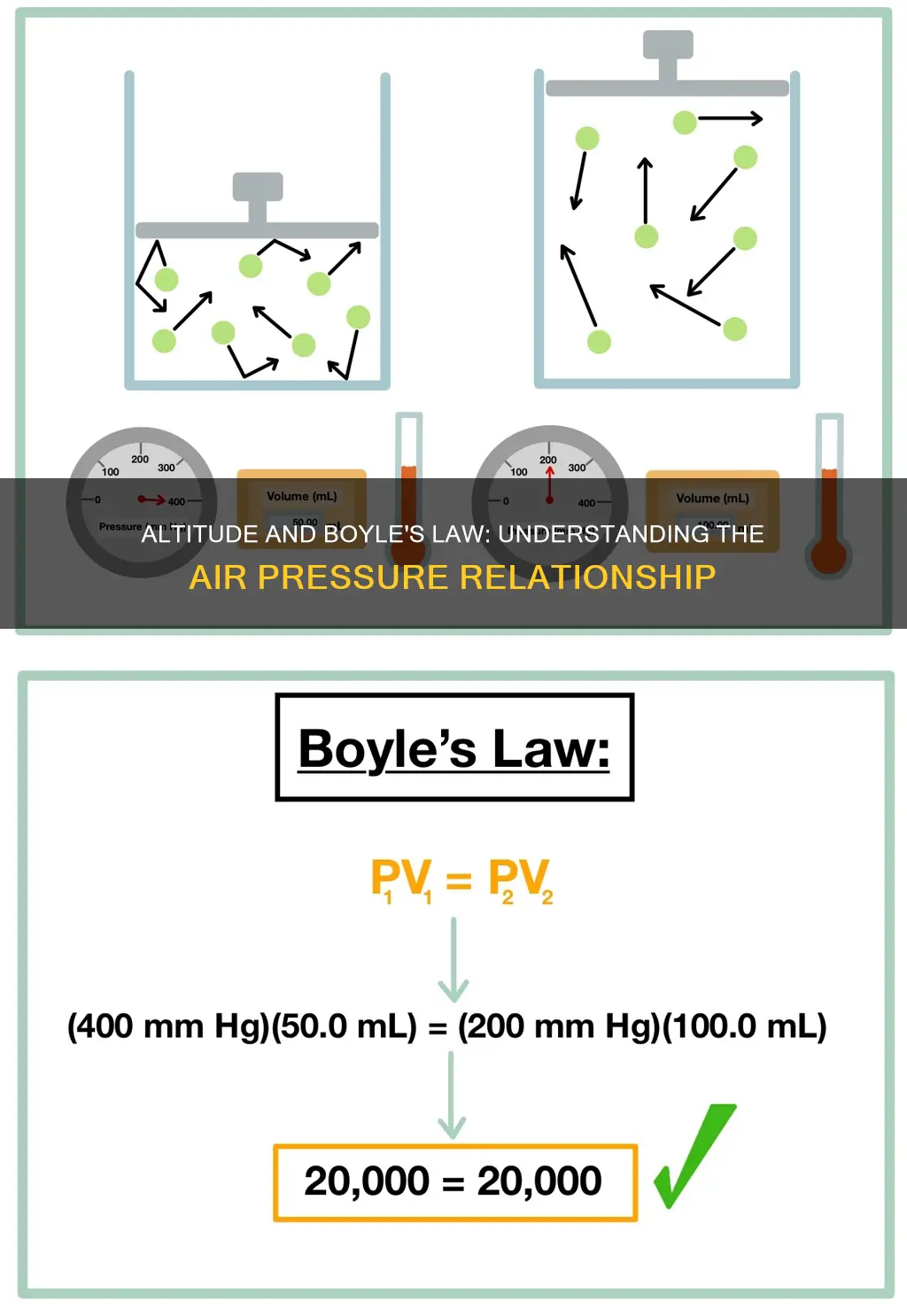
| What is the pressure-volume relationship? | The product of the initial pressure and the initial volume of a gas is equal to the product of its final pressure and final volume (at constant temperature and number of moles). |
| What is the mathematical representation of the pressure-volume relationship? | P1V1 = P2V2 |
| What is an example of Boyle's Law? | If a scuba diver rapidly ascends from a deep zone towards the surface of the water, the decrease in pressure can cause the gas molecules in their body to expand. |
What You'll Learn

Gas expansion in bodily injuries or diseases
Boyle's law states that, "at a constant temperature, the volume of a given gas is inversely proportional to the pressure to which it is subjected". In other words, when the temperature of a given mass of confined gas is constant, the product of its pressure and volume is also constant.
This law is particularly relevant when considering the human body, which maintains a constant temperature. As altitude increases, the gas inside a closed space is subject to expansion. This has important implications when dealing with bodily injuries or diseases. For instance, in the case of a pneumothorax, a condition where air escapes into the space between the lung and the chest wall, the expansion of gas can be dangerous. This is because the pressure surrounding the lung increases, which can lead to a collapse of the lung.
Boyle's law also has implications for bowel obstructions. For example, in the case of bowel gas in the area of the uterus, the expansion of gas may hasten the delivery process as the body attempts to relieve the surrounding pressure created by the gas expansion.
Additionally, Boyle's law explains the mechanism of air exchange between the atmosphere and the lungs. As the chest expands due to the movement of the thoracic cage and diaphragm, the volume of the chest cavity increases, leading to a decrease in pressure. This causes ambient air to rush in until the pressure equalizes.
In summary, Boyle's law is a critical concept in understanding the effects of altitude and pressure changes on the human body, particularly in cases of bodily injuries or diseases involving gas expansion.
Hubble's Law: Universal or Group-Specific?
You may want to see also

Gas exchange between the atmosphere and the lungs
Boyle's law states that, "at a constant temperature, the volume of a given gas is inversely proportional to the pressure to which it is subjected". In other words, as the volume of a gas increases, its pressure decreases, and vice versa, when the temperature is held constant. This law can be applied to the human body, specifically to the breathing system.
The human lungs are composed of branching airways that terminate in respiratory bronchioles and alveoli, which participate in gas exchange. Gas exchange occurs in the respiratory zone of the lung, where alveoli are present. The respiratory zone includes respiratory bronchioles, alveolar ducts, alveolar sacs, and alveoli. Thin alveolar septa separate adjacent alveoli, which have connections via small openings called pores of Kohn. These pores allow for collateral airflow and equalization of pressure between alveoli.
The control of opening or closing alveoli to regulate ventilation occurs at the alveolar duct. The alveolar septum contains type I pneumocytes that line the alveoli, as well as type II pneumocytes that secrete surfactant to decrease alveolar surface tension. Alveolar macrophages, also known as dust cells, defend the lungs against pathogens and irritants.
Gas exchange in the alveoli occurs primarily by diffusion. Oxygen moves from the alveoli to the bloodstream, while carbon dioxide passes from the blood to the lungs. This exchange happens between the alveoli and a network of tiny blood vessels called capillaries, which are located in the walls of the alveoli. The walls of the alveoli share a membrane with the capillaries, allowing oxygen and carbon dioxide to diffuse or move freely between the respiratory system and the bloodstream.
Three processes are essential for the transfer of oxygen from the outside air to the blood flowing through the lungs: ventilation, diffusion, and perfusion. Ventilation refers to the flow of air into and out of the alveoli, while perfusion refers to the flow of blood to alveolar capillaries. Diffusion is the spontaneous movement of gases between the alveoli and capillaries without the use of any energy by the body.
According to Fick's law of diffusion, diffusion of a gas across the alveolar membrane increases with an increased surface area of the membrane, increased alveolar pressure difference, increased solubility of the gas, and decreased membrane thickness. The exchange of both oxygen and carbon dioxide is perfusion-limited, meaning that diffusion reaches equilibrium one-third of the way through the capillary/alveolar interface.
Collective changes in ventilation and perfusion in the lungs are measured using the ratio of ventilation to perfusion (V/Q). Changes in the V/Q ratio can affect gas exchange and contribute to hypoxemia. A number of clinical conditions, such as pulmonary embolism and asthma, can cause V/Q mismatches, resulting in impaired gas exchange and hypoxemia.
In summary, Boyle's law helps explain the mechanism of gas exchange between the atmosphere and the lungs by demonstrating the relationship between pressure and volume in a confined gas. The human respiratory system utilises ventilation, diffusion, and perfusion to facilitate the exchange of oxygen and carbon dioxide between the alveoli and capillaries in the lungs.
Usury Laws: Do They Apply to Individuals?
You may want to see also

Gas expansion in equipment designed to hold gas
The expansion of gas in closed cavities within the body, such as the sinuses or middle ear, can also cause discomfort or pain during altitude or pressure changes. Additionally, conditions such as pneumothorax or bowel obstruction can be influenced by gas expansion at higher altitudes. For example, a pneumothorax with a volume of 1500 mL at sea level can increase to a volume of 1688 mL at an altitude of 1 km, assuming a constant temperature. This expansion can have significant implications for patient care and treatment.
The effects of gas expansion in equipment designed to hold gas can also be observed in scuba diving. If a scuba diver ascends too quickly from a deep zone towards the surface, the decrease in pressure can cause the gas molecules in their body to expand. This expansion can lead to the formation of gas bubbles, which can cause damage to the diver's organs and even result in death. This phenomenon is known as decompression sickness and highlights the importance of understanding gas laws when dealing with changes in altitude.
Furthermore, gas expansion can impact the functionality of equipment used in aviation and aerospace contexts. For example, during a flight, the expansion of a sealed bag of potato chips can be observed due to the decrease in ambient pressure. This effect is not limited to food items but can also affect other gas-filled equipment on board. Understanding and predicting these changes are crucial for the safe operation of aircraft and the well-being of passengers and crew.
In summary, gas expansion in equipment designed to hold gas is a significant factor to consider when dealing with changes in altitude. Boyle's law provides a fundamental understanding of the relationship between pressure and volume, which helps explain the behaviour of gases in various contexts, including human physiology, aviation, and scuba diving. By applying this knowledge, we can monitor and adjust equipment and procedures to ensure the safety and effectiveness of systems that rely on the containment of gases.
US Laws in Puerto Rico: Who's in Charge?
You may want to see also

Gas expansion in closed cavities within the body
Boyle's law states that, "at a constant temperature, the volume of a given gas is inversely proportional to the pressure to which it is subjected". In other words, as altitude increases, the gas inside a closed space is subject to expansion.
This has several implications for the human body, which contains several closed cavities, including the middle ear, the sinuses, the lungs, the gastrointestinal tract, and the teeth.
Middle Ear
The middle ear is a closed space that can be affected by changes in air pressure. When a person ascends to higher altitudes, the pressure in the middle ear decreases, and the volume of gas in the ear expands. Normally, air is transmitted through the Eustachian tube to equalise the pressure. However, on descent, when the ambient pressure increases, a relative vacuum forms in the middle ear, and the Eustachian tube may not open without conscious assistance. This can lead to a condition called barotitis media, or ear block, which is more common on descent. Inflammation or congestion due to allergies or an upper respiratory infection can worsen this condition. In extreme cases, the eardrum can rupture.
Sinuses
The sinuses are another set of closed cavities in the human body that can be affected by changes in air pressure. Similar to barotitis media, a negative pressure gradient in the sinuses can cause mucosal congestion and even bleeding as fluids are pulled from deeper tissues to the surface. This can result in considerable pain and, in some cases, tissue damage.
Lungs
As altitude increases, the gas inside the lungs expands. Usually, this air is passively expired, but if the expansion is too rapid, lung tissue can tear, leading to conditions such as pneumothorax, pneumomediastinum, or arterial gas embolism.
Gastrointestinal Tract
The gastrointestinal tract is a continuous space from the mouth to the rectum and anus. While it has two openings, decreasing pressure during ascent can still cause symptoms as the volume of gastrointestinal gas expands. This expansion can stimulate pain receptors and cause abdominal discomfort. In some cases, it can even interfere with respiration by preventing the diaphragm from fully descending during inhalation. Consuming gas-producing foods, such as beans, broccoli, or carbonated drinks, before flying or scuba diving can exacerbate these issues.
Teeth
Gas can become trapped in teeth during dental procedures, such as fillings, or as a result of infectious processes such as tooth abscesses. In pilots or scuba divers, the expansion or contraction of these small pockets of air during changes in altitude can lead to incredible pain or even tooth fracture. This condition is known as dental barotrauma or barodontalgia.
Understanding ADA Compliance for Cell Phones
You may want to see also

Gas expansion in the human breathing system
Boyle's law states that, "at a constant temperature, the volume of a given gas is inversely proportional to the pressure to which it is subjected". In other words, when the volume of a gas increases, its pressure decreases, and vice versa, as long as the temperature remains the same. This law can be applied to the human breathing system to explain how inhalation and exhalation occur.
The human respiratory system consists of a group of organs and tissues that work together to facilitate breathing. Air is moved into the lungs (inhalation) and out of the lungs (exhalation) to facilitate gas exchange, primarily to bring in oxygen and flush out carbon dioxide. The lungs themselves do not have the capacity to inflate or deflate; instead, this is achieved through an increase or decrease in the volume of the thoracic cavity, which is mainly achieved through the contraction of the diaphragm.
When the diaphragm contracts, the volume of the thoracic cavity increases, causing a decrease in pressure within the chest cavity. This decrease in pressure creates a pressure difference between the air inside the lungs and the environmental air pressure, causing air to rush into the lungs (inhalation) until the pressure is equalised. Conversely, when the diaphragm relaxes and returns to its resting position, the volume of the thoracic cavity decreases, causing an increase in pressure within the chest cavity. This, in turn, creates a pressure difference that causes air to be pushed out of the lungs (exhalation) until pressure equilibrium is restored.
Boyle's law can be used to explain the effects of altitude on gases in closed cavities within the body. As altitude increases, ambient pressure decreases, and according to Boyle's law, volume expansion occurs in enclosed spaces. This has implications for bodily injuries or diseases such as pneumothorax or bowel obstruction. For example, in one study, a 40 mL pneumothorax increased in volume by up to 16% at 1.5 km (approximately 5000 feet) above sea level. It is estimated that a closed volume of gas in the human body, such as a bulla, can expand by up to 30% when ascending from sea level to an altitude of 2.5 km (approximately 8200 feet).
Additionally, at high altitudes, a greater volume of air must be inhaled to breathe in the same amount of oxygen as at sea level. This is because the atmospheric pressure and the concentration of oxygen in the air decrease with increasing altitude. As a result, the respiratory minute volume, or the volume of air breathed in or out per minute, tends to increase automatically to compensate for the decrease in oxygen concentration.
Antitrust Laws: Nonprofit Sector's Friend or Foe?
You may want to see also
Frequently asked questions
Boyle's Law is a gas law that describes the relationship between the pressure and volume of a confined gas. It states that, at a constant temperature, the volume of a given mass of a dry gas is inversely proportional to its pressure.
As altitude increases, the pressure exerted on a gas decreases, and the volume of the gas increases. This is because the gas expands to occupy a greater volume at lower pressures. This principle can be applied to understand the effects of altitude on gases in closed cavities within the body, such as the sinuses or middle ear.
Boyle's Law assumes that the temperature and quantity of gas remain constant. It also applies to low pressure and not high pressure, as gases behave like ideal gases at high pressure.







