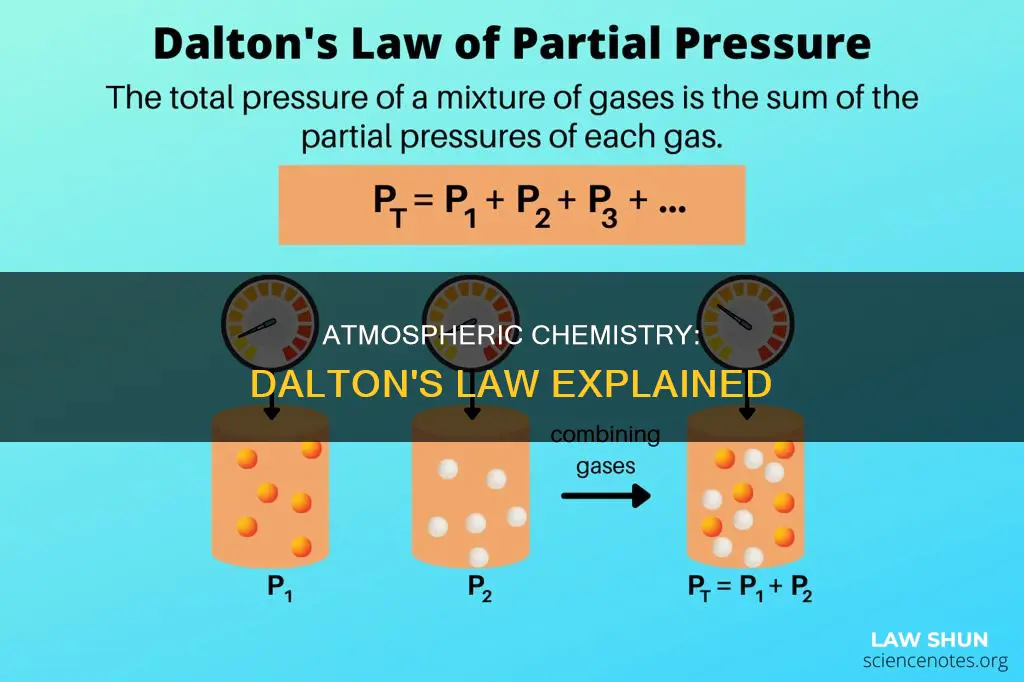
Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the gases in the mixture. This empirical law was observed by John Dalton in 1801 and published in 1802.
Mathematically, the pressure of a mixture of non-reactive gases can be defined as:
> p_total = p_1 + p_2 + p_3 + ... + p_n
Where p1, p2, pn represent the partial pressures of each component.
Dalton's Law can be applied to the atmosphere by considering the various layers of the atmosphere and the individual pressures they exert on the Earth's surface. For example, we can calculate the combined pressure exerted on the Earth by adding the pressures of the ionosphere and the lithosphere. This law is also used to find the pressures of pure gases such as hydrogen or helium.
What You'll Learn
- Dalton's Law can be used to calculate the total pressure exerted by the mixture of gases in the atmosphere
- The Law of Partial Pressures can be used to determine the pressure exerted by each gas in the atmosphere, as if it alone occupied the volume
- The relative concentration of gases in the atmosphere remains constant, even as pressure and volume change
- Dalton's Law can be used to calculate the pressure exerted by water vapour in the atmosphere
- The Law of Partial Pressures can be used to determine the percentage of each gas in the air we breathe

Dalton's Law can be used to calculate the total pressure exerted by the mixture of gases in the atmosphere
Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of gases is You may want to see also Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. In other words, each gas in the mixture exerts the same pressure as it would if it alone occupied the volume. This law was observed by John Dalton in 1801 and published in 1802. Mathematically, the pressure of a mixture of non-reactive gases can be defined as: \[ p_\text{{total}} = p_1 + p_2 + p_3 + ... + p_n \] Where p1, p2, etc. represent the partial pressures of each gas in the mixture. For example, let's consider the Earth's atmosphere, which is composed of several gases, including nitrogen (78%), oxygen (21%), argon (0.9%), and carbon dioxide (0.04%). Using Dalton's Law, we can calculate the pressure exerted by each of these gases in the atmosphere. If the total pressure exerted by the mixture of gases in the atmosphere is P_total, then the pressure exerted by nitrogen (N2) can be calculated as: \[ P_\text{{N2}} = P_\text{{total}} \times \frac{{\text{{Mole fraction of N}}_2}}{{\text{{Mole fraction of total mixture}}}} \] Similarly, we can calculate the pressure exerted by oxygen (O2), argon (Ar), and carbon dioxide (CO2) using their respective mole fractions in the atmosphere. It is important to note that Dalton's Law assumes ideal gas behaviour, and real gases may deviate from this law at high pressures or low temperatures. However, the Earth's atmosphere can be approximated as an ideal gas under standard conditions, making Dalton's Law a useful tool for understanding the partial pressures of gases in the atmosphere. You may want to see also Dalton's Law, also known as the Law of Partial Pressures, was observed by John Dalton in 1801 and published in 1802. It states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of the individual gases. This can be expressed mathematically as: > p_total = p_1 + p_2 + p_3 + ... + p_n Where p1, p2, etc., represent the partial pressures of each component gas. The air in the Earth's atmosphere is a mixture of nitrogen, water vapour, oxygen, carbon dioxide, and other trace gases. The concentration of water vapour in the atmosphere varies from around 10 parts per million in the coldest portions of the atmosphere to as much as 5% in hot, humid air masses. The relative concentration of gases remains constant until about 10,000 metres (33,000 feet). Dalton's Law is only completely accurate for ideal gases. Real gases do not strictly follow Dalton's Law, and the deviation increases with pressure. At high pressures and low temperatures, gases are more likely to react and change the pressure of the system. You may want to see also Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases. This law was observed by John Dalton in 1801 and published in 1802. It is related to the ideal gas laws. Mathematically, the pressure of a mixture of non-reactive gases can be defined as: \p_{total} = p_1 + p_2 + p_3 + ... + p_n Where p1, p2, ..., pn represent the partial pressures of each component. Dalton's Law can be applied to the atmosphere, which is a mixture of various gases, including nitrogen, water vapour, oxygen, carbon dioxide, and other minor gases. According to Dalton's Law, the total atmospheric pressure is the sum of the partial pressures exerted by each of these gases. Water vapour in the atmosphere contributes to the total atmospheric pressure. By knowing the partial pressure of water vapour and the total atmospheric pressure, we can use Dalton's Law to calculate the partial pressures exerted by the other gases in the atmosphere. For example, if we know that the partial pressure of water vapour is 23.8 mmHg and the total atmospheric pressure is 754 mmHg, we can calculate the partial pressure of another gas, such as hydrogen. By subtracting the water vapour pressure from the total pressure, we find the partial pressure of hydrogen: \p_{H_2} = p_{total} - p_{H_2O} = 754 mmHg - 23.8 mmHg = 721.6 mmHg Converting this to atm: \p_{H_2} = 721.6 mmHg \times \frac{1 atm}{760 mmHg} = 0.949 atm So, the partial pressure of hydrogen in this example is 0.949 atm. In summary, Dalton's Law allows us to understand the contribution of each gas to the total atmospheric pressure, including water vapour. By knowing the partial pressure of water vapour and the total atmospheric pressure, we can calculate the partial pressures of other gases in the atmosphere. You may want to see also Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture. This empirical law was observed by John Dalton in 1801 and published in 1802. Mathematically, this can be expressed as: P_total = P1 + P2 + P3 + ... Where P1, P2, P3, etc. represent the partial pressures of each gas. According to Dalton's Law, the percentage of each gas in the air we breathe contributes to the total atmospheric pressure. This contribution depends on the amount of each gas present in the air. To determine the percentage of each gas in the air we breathe, we can use the following steps: By following these steps and using the principles of Dalton's Law, we can determine the percentage of each gas in the air we breathe and how it contributes to the total atmospheric pressure. You may want to see also Dalton's Law, also known as the Law of Partial Pressures, states that the total pressure exerted by a mixture of non-reacting gases is equal to the sum of the partial pressures of each gas in the mixture. Dalton's Law can be applied to the various layers of the atmosphere to determine the individual pressures each layer exerts on the Earth's surface. For example, we can calculate the combined pressure exerted on the Earth by adding the pressures of the ionosphere and lithosphere. Dalton's Law is significant in respiratory physiology as it helps explain how gases dissolve in the alveoli and bloodstream during gas exchange. It also helps determine the rate at which gases diffuse across the alveolar membranes, influencing the process of respiration.The Law and the Rich: Who's Exempt?

The Law of Partial Pressures can be used to determine the pressure exerted by each gas in the atmosphere, as if it alone occupied the volume
Stark Law and Ambulatory Surgery Centers: Understanding the Connection

The relative concentration of gases in the atmosphere remains constant, even as pressure and volume change
Applying for a Lawful Development Certificate: A Guide

Dalton's Law can be used to calculate the pressure exerted by water vapour in the atmosphere
Internship Wage Laws: Minimum Wage Compliance

The Law of Partial Pressures can be used to determine the percentage of each gas in the air we breathe
Suppressor Self-Manufacture: Legal or Not?
Frequently asked questions







