Charles' Law, also known as the Law of Volume, is an experimental gas law that explains the relationship between the volume of a given mass of gas and its temperature. It states that volume and temperature are directly proportional to each other. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This law has numerous applications in everyday life, from the simple act of baking to more complex processes like hot air ballooning and automotive engineering. Understanding Charles' Law can help us make sense of the science behind common phenomena and improve our daily decision-making.
| Characteristics | Values |
|---|---|
| Name | Charles's Law (also known as the Law of Volumes) |
| Discovery | Formulated by French physicist Jacques Charles in the 1780s |
| Application | Describes how gases expand when heated and contract when cooled, at constant pressure |
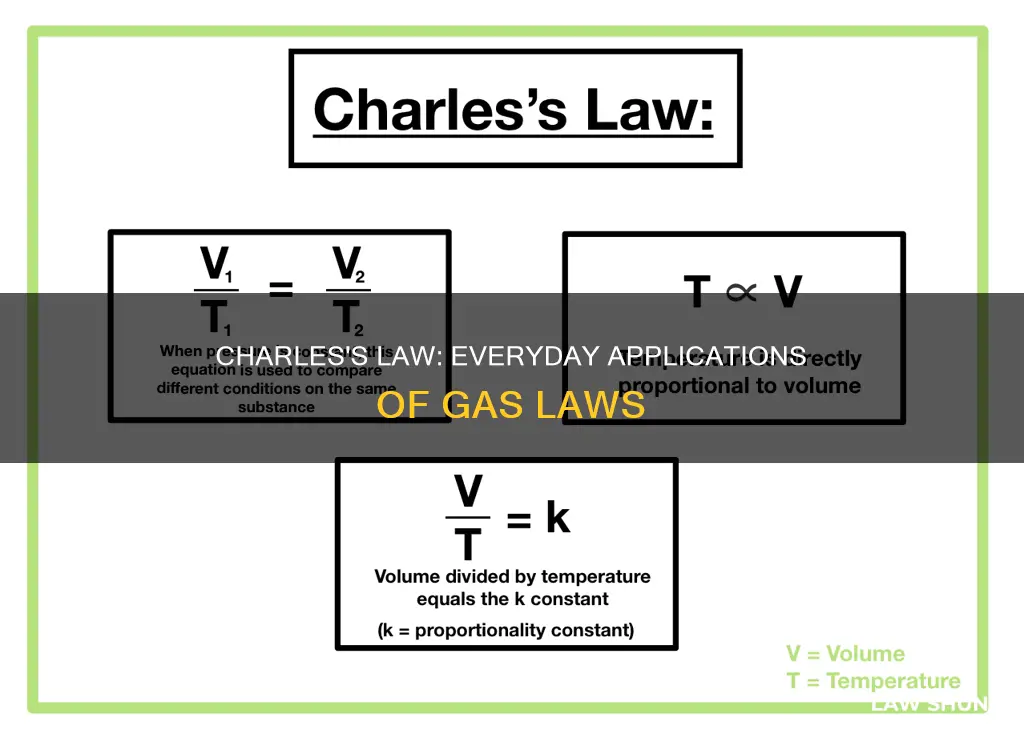
| Formula | V ∝ T, V/T = k, V = kT, V1/T1 = V2/T2, V2/V1 = T2/T1, V1T2 = V2T1 |
| Variables | V = volume of gas, T = temperature of gas (in Kelvin), k = constant for a particular pressure and amount of gas |
| Examples | Pop-up turkey thermometers, car engines, hot air balloons, balloons in different weather |
What You'll Learn

How does Charles's Law apply to hot air balloons?
Charles's Law, discovered by French physicist Jacques Charles in the 1780s, states that when the pressure of a gas is held constant, increasing its temperature increases its volume. Conversely, decreasing the temperature of a gas decreases its volume.
This law is directly applicable to hot air balloons. The gas inside a hot air balloon is heated, causing it to expand. As the balloon has a fixed volume, the extra volume of the gas flows out of the hole in the bottom of the balloon. With less gas inside the balloon, its density decreases. According to Archimedes' Principle, an object floats when it weighs less than the fluid it displaces. Therefore, when the air inside the balloon is heated, the net weight of the balloon plus the hot air becomes less than the weight of the same volume of cold air, and the balloon rises. Conversely, when the gas inside the balloon cools down, its volume decreases, cold air moves in through the hole, the weight of the balloon plus the air increases, and the balloon starts to sink.
The gas used in hot air balloons is typically hydrogen or helium, both of which are lighter than air. By adding heat to the air inside the balloon, the molecules move further apart from each other, and the total density (mass per unit of volume) of the balloon and the air inside it decreases. When the density of the balloon is less than the density of the outside air, the balloon rises.
Securities Laws: Private Companies' Obligations and Exemptions
You may want to see also

How does Charles's Law apply to car tyres?
Charles's Law, also known as the law of volumes, states that the volume of an ideal gas is directly proportional to its absolute temperature, provided the pressure is held constant. This law was formulated by French physicist Jacques Charles in 1780.
Now, how does this apply to car tyres? Well, the air inside a car tyre behaves like an ideal gas. When the temperature drops, the volume of the air inside the tyre decreases, leading to a decrease in pressure. This is why, during winter, car tyres tend to deflate. The opposite happens when the temperature increases, causing the air inside the tyre to expand and leading to higher pressure.
As a result, it is recommended to check your car tyre pressure when the tyres are cold. Driving heats up the tyres, causing the air inside to expand and increasing the pressure. So, if you check the pressure when the tyres are warm, you may get the false impression that your tyres are overinflated.
Therefore, Charles's Law is important to keep in mind when maintaining proper tyre pressure, which is crucial for optimal traction, fuel economy, and the overall safety of your vehicle.
Open Carry Laws: Rifles Included or Excluded?
You may want to see also

How does Charles's Law apply to sports performance?
Charles's Law, also known as the law of volumes, states that the volume of a fixed amount of gas held at a constant pressure is directly proportional to its absolute temperature. This means that as the temperature of a gas increases, so does its volume, and vice versa. This law was formulated by French physicist Jacques Charles in the 1780s.
Now, how does this apply to sports performance? Well, let's consider the impact of temperature on the human body and its ability to perform athletically.
Firstly, Charles's Law helps explain why athletes may struggle to perform in freezing temperatures. As the law states, the volume of a gas is directly proportional to its temperature. So, when the temperature decreases, the volume of a gas also decreases. This is relevant to sports performance because, in colder temperatures, the capacity of the human lung decreases, making it harder to breathe and, therefore, perform physical activities. This is why athletes often prefer warmer conditions for competitions, as they can breathe more easily and their lungs can function at full capacity.
Additionally, Charles's Law can explain the impact of temperature on sports equipment. For example, a basketball will shrink when taken outside in cold weather, affecting its performance. Similarly, the pressure in car tires needs to be checked regularly, especially in cold temperatures, as the gas molecules inside the tires slow down and take up less space, reducing the overall pressure. This can affect the handling and performance of the vehicle.
Charles's Law also applies to the human body's internal processes. For instance, the law can explain the function of pop-up turkey thermometers. As the meat cooks and the internal temperature increases, the gas inside the thermometer expands, causing the plunger to pop. This principle can be applied to the human body, specifically to processes that involve gas production and expansion within the body.
In summary, Charles's Law helps us understand the relationship between temperature and volume in sports equipment and the human body. It explains how temperature variations can impact athletic performance by affecting breathing, equipment functionality, and internal body processes.
Skype Surveillance: Are Your Calls and Chats Being Watched?
You may want to see also

How does Charles's Law apply to cooking?
Charles's Law of Ideal Gases, named after Jacques Charles, states that the volume of a fixed mass of gas is directly proportional to its temperature. This means that as the temperature increases, the volume of the gas also increases, and vice versa. This law applies to ideal gases held at a constant pressure, where only the volume and temperature are allowed to change.
In cooking, Charles's Law is particularly relevant in understanding the behaviour of gases during various culinary processes. For instance, when baking bread or cakes, the carbon dioxide trapped in the fermented dough expands as the temperature in the oven increases, resulting in fluffy and airy textures. Similarly, in the preparation of soufflés, the delicate batter rises due to the expansion of gas bubbles, but it may collapse during cooling as the volume of gas decreases.
Additionally, Charles's Law explains the functionality of pressure cookers. When the stove heats the cooker, the pressure and internal temperature increase, leading to a higher boiling point for water. This accelerated cooking process is especially advantageous at high altitudes, where water boils at lower temperatures.
The law also highlights the importance of temperature control in cooking. For example, when removing baked goods from the oven too early, they may collapse due to the loss of gas molecules and the subsequent decrease in volume. Understanding Charles's Law can help cooks optimise their recipes and prevent undesirable outcomes, such as collapsed cakes or deflated pastries.
Furthermore, Charles's Law underscores the potential dangers associated with certain kitchen products. Aerosol and deodorant spray cans, when exposed to increased temperatures, can burst due to the expansion of compressed gases. This knowledge is crucial for the safe handling and storage of such products.
Volunteer Rights: Anti-Discrimination Laws and Their Applicability
You may want to see also

How does Charles's Law apply to human lungs?
Charles's Law, also known as the law of volumes, is an experimental gas law that describes how gases tend to expand when heated. The law was formulated by scientist Jacques Charles in the 1780s.
Charles's Law states that the volume of a gas is directly proportional to its absolute temperature, provided the pressure remains constant. This can be written as:
V ∝ T
Or
V = kT
Where V is the volume of the gas, T is the temperature of the gas (measured in kelvins), and k is a constant for a particular pressure and amount of gas.
This law has implications for human lungs, which are air-filled organs responsible for respiration. When we inhale cold air, it passes through our respiratory tract and warms up, causing it to expand and increase in volume as per Charles's Law. This increase in volume means that we need to take shorter breaths in cold weather to inhale the same amount of air.
The change in volume of inhaled air due to temperature differences can be calculated using the equation:
V2 = V1 × T2/T1
Where V1 is the initial volume of cold air, T2 is the temperature of the body (around 37°C), and T1 is the external temperature.
For example, if it is -10°C outside and we want to inhale 500 mL of air at body temperature, we would need to inhale 420 mL of cold air, as the volume increases when it reaches the warmer temperature inside our lungs.
Charles's Law also explains why physical activities like jogging become more difficult on freezing winter days. As the temperature of the air decreases, the volume of air in our lungs decreases according to the law, causing our lungs to shrink and making it harder to breathe.
Therefore, Charles's Law affects the volume of air we can inhale and has a noticeable impact on our breathing, especially in cold weather.
HOA and the Law: Understanding "Under the Color of Law
You may want to see also