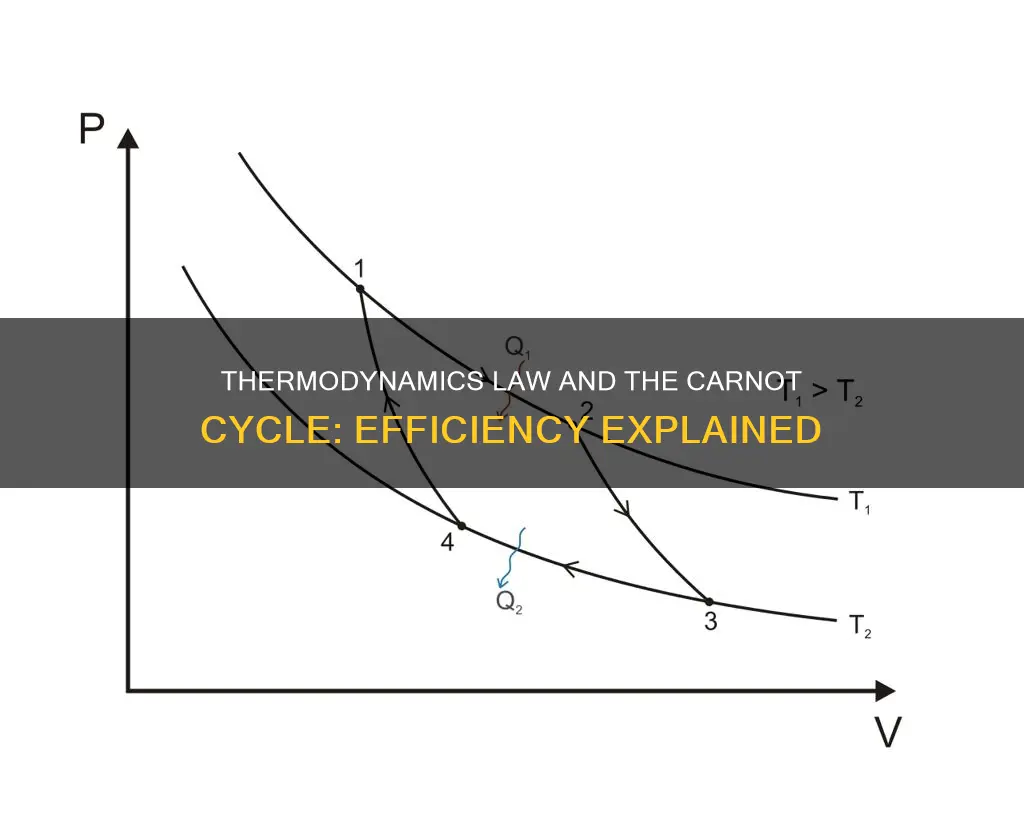
The Carnot cycle, proposed by Sadi Carnot in 1824, is a fundamental thermodynamic cycle that forms the basis for understanding engines. It is a theoretical cycle that describes the most efficient cyclical process possible for heat engines, and any engine employing the Carnot cycle is known as a Carnot engine. The cycle consists of four processes: two reversible isothermal processes and two reversible adiabatic processes. The first process is an isothermal expansion, where heat is transferred from a hot temperature reservoir at a constant temperature, allowing the gas to expand and do work on the surroundings. This is followed by an adiabatic expansion, where the gas continues to expand and do work, causing a decrease in temperature. The third process is an isothermal compression, where the gas is compressed and heat is transferred to the low-temperature reservoir. Finally, an adiabatic compression occurs, where the gas is further compressed, causing a rise in temperature. The Carnot cycle has important implications for the second law of thermodynamics, as it demonstrates that a heat engine cannot be 100% efficient due to inevitable heat transfer to the environment.
What You'll Learn
- The Carnot cycle is a theoretical limit of macroscopic heat engines
- The Carnot cycle is a cyclical process that uses only reversible processes
- The Carnot cycle is a closed power cycle
- The Carnot cycle is a reversible thermodynamic cycle
- The Carnot cycle is a useful model for advancing the development of the diesel engine

The Carnot cycle is a theoretical limit of macroscopic heat engines
The Carnot cycle is a theoretical thermodynamic cycle proposed by French physicist Sadi Carnot in 1824. It is a model of a thermodynamic system for a heat engine, and it forms the basis of the second law of thermodynamics. The Carnot cycle consists of four processes: a reversible isothermal gas expansion, a reversible adiabatic gas expansion, a reversible isothermal gas compression, and a reversible adiabatic gas compression.
The Carnot cycle is the most efficient engine possible, assuming the absence of incidental wasteful processes such as friction and the absence of heat conduction between different parts of the engine. The efficiency of the Carnot engine is defined as the ratio of energy output to energy input.
The Carnot cycle is a theoretical construct, and an actual Carnot engine would be impractical to build. However, on a practical human scale, the Carnot cycle has proven valuable as a model for developing technologies such as the diesel engine. On a macroscopic scale, the limitations imposed by the model's assumptions make it impractical and incapable of doing any work.
Therefore, the Carnot engine can be considered the theoretical limit of macroscopic heat engines, rather than a practical device that could be built.
Laws for All: Citizens and Noncitizens
You may want to see also

The Carnot cycle is a cyclical process that uses only reversible processes
The Carnot cycle is a theoretical cyclical process that uses only reversible processes. It was first proposed by French physicist Sadi Carnot in 1824 as an ideal thermodynamic cycle. The Carnot cycle provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work.
In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs, a hot reservoir and a cold reservoir. This transferred energy is then converted into work done by the system. The cycle is reversible, and entropy is conserved, merely transferred between the thermal reservoirs and the system without gain or loss.
The Carnot cycle consists of four processes: a reversible isothermal gas expansion process, a reversible adiabatic gas expansion process, a reversible isothermal gas compression process, and a reversible adiabatic gas compression process. During the isothermal processes, the system either absorbs or releases heat while maintaining a constant temperature. In the adiabatic processes, the system is thermally insulated, and there is no heat transfer.
The efficiency of a Carnot engine is defined as the ratio of the energy output to the energy input. The Carnot cycle has the greatest efficiency possible for an engine based on the assumption that there are no incidental wasteful processes, such as friction, and no conduction of heat between different parts of the engine at different temperatures.
HIPAA Laws: Do They Apply to Spouses?
You may want to see also

The Carnot cycle is a closed power cycle
The Carnot cycle is a reversible cycle, and it consists of four processes: two isothermal and two adiabatic. In a Carnot cycle, a system or engine transfers energy in the form of heat between two thermal reservoirs, a hot and a cold reservoir, and a part of this transferred energy is converted to the work done by the system.
The first process is an isothermal expansion, where heat is transferred from the hot temperature reservoir at a constant temperature to the gas, which expands and does work on the surroundings. The second process is an isentropic (reversible adiabatic) expansion of the gas, where it is thermally insulated from both reservoirs and continues to expand and do work, causing a loss of internal energy and a decrease in temperature.
The third process is an isothermal compression, where heat is transferred to the low-temperature reservoir at a constant temperature, and the surroundings do work on the gas, causing a loss of heat. The fourth and final process is an isentropic compression, where the gas is once again thermally insulated, and the surroundings do work to compress the gas, causing its temperature to rise back to the original temperature.
The Carnot cycle is the most efficient cyclical process possible, and any engine using the Carnot cycle, with only reversible processes, is known as a Carnot engine. However, in reality, no engine achieves Carnot's theoretical maximum efficiency due to dissipative processes such as friction.
Understanding Landlord Laws: Paperwork or Not?
You may want to see also

The Carnot cycle is a reversible thermodynamic cycle
The Carnot cycle, proposed by French physicist Sadi Carnot in 1824, is a reversible thermodynamic cycle. It is an idealised thermodynamic cycle that provides an upper limit on the efficiency of any classical thermodynamic engine during the conversion of heat into work.
The Carnot cycle consists of four processes: a reversible isothermal gas expansion process, a reversible adiabatic gas expansion process, a reversible isothermal gas compression process, and a reversible adiabatic gas compression process. These processes allow the cycle to transfer energy in the form of heat between two thermal reservoirs at different temperatures, with a portion of this transferred energy being converted into work done by the system.
The Carnot cycle is reversible, meaning that the entropy is conserved and merely transferred between the thermal reservoirs and the system without any net gain or loss. This is a crucial aspect of the cycle, as it ensures that the efficiency of the engine is maximised.
The efficiency of a Carnot engine is defined as the ratio of the energy output to the energy input. The cycle's reversible nature ensures that the engine operates at the greatest possible efficiency of any heat engine operating between the same two temperatures.
Overall, the Carnot cycle is a reversible thermodynamic cycle that serves as a theoretical model for maximising the efficiency of heat engines.
California Evidence Code: Admin Law Proceeding Applicability
You may want to see also

The Carnot cycle is a useful model for advancing the development of the diesel engine
The Carnot cycle is particularly useful for the development of the diesel engine because it provides a framework for understanding and improving the engine's efficiency. The efficiency of a Carnot engine is defined as the ratio of the energy output to the energy input. Specifically, it is given by the equation:
> Efficiency = 1 - (Temperature of cold reservoir / Temperature of hot reservoir)
This equation highlights the importance of the temperature difference between the hot and cold reservoirs in determining the efficiency of the engine. By maximizing this temperature difference, the efficiency of the engine can be increased.
In addition to providing a theoretical framework for understanding efficiency, the Carnot cycle also offers insights into the design and operation of heat engines. For example, the Carnot cycle emphasizes the importance of reversible processes, which are free of dissipative factors such as friction and turbulence. By minimizing these dissipative factors, the efficiency of the engine can be further enhanced.
The Carnot cycle has been applied to the development of the diesel engine, with Rudolf Diesel patenting an internal combustion engine inspired by the Carnot cycle in 1892. While Diesel recognized that a true Carnot engine was unattainable, he believed he could create a working approximation. Although Diesel's initial attempts to create an isothermal combustion engine were unsuccessful, his efforts laid the foundation for the practical Diesel engine that evolved over the next 25 years. By 1969, the Diesel engine had achieved 40% efficiency, showcasing the impact of the Carnot cycle on its development.
Virginia Seat Belt Law: Rear Seats and Exemptions
You may want to see also







