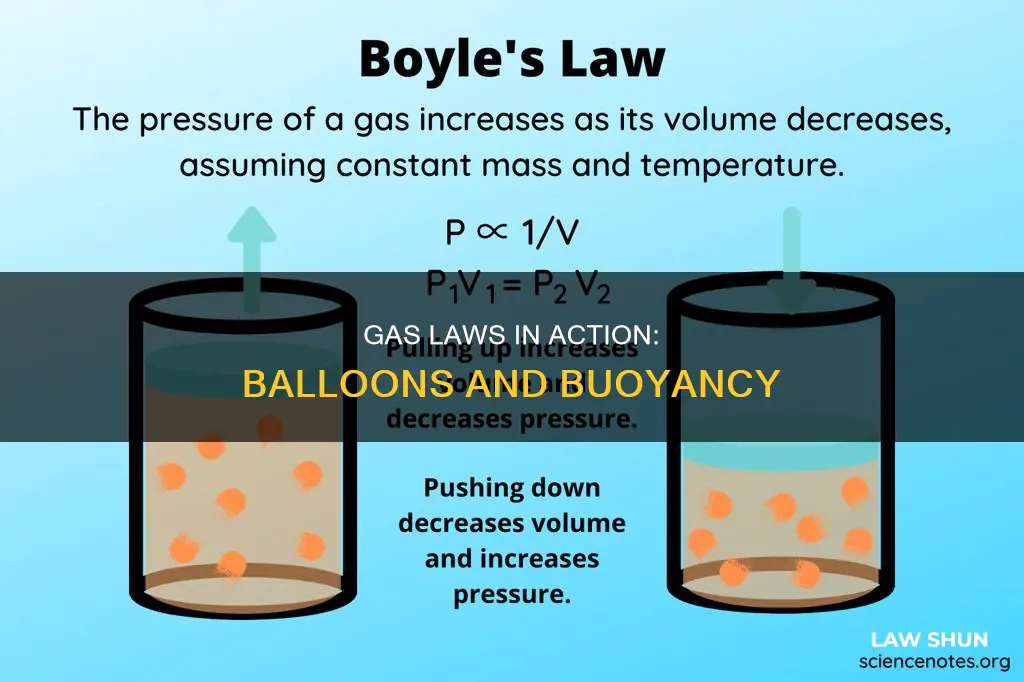
The behaviour of gases can be described by several laws based on experimental observations of their properties. One of these laws is Boyle's Law, which states that the volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant. This means that as the pressure on a gas increases, its volume decreases, and vice versa. This law is particularly relevant to understanding the behaviour of balloons, as it explains why weather balloons get larger as they rise through the atmosphere to regions of lower pressure.
| Characteristics | Values |
|---|---|
| Gas Law | Boyle's Law |
| Relationship | Inverse relationship between pressure and volume |
| Equation | PV = constant |
| V ∝ 1/P | |
| V = constant/P | |
| Statement | At constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure |
| At constant temperature, the volume of a given amount of gas is inversely proportional to its pressure | |
| Discovery | Discovered by Robert Boyle |
| Discovered in the 17th and 18th centuries |
What You'll Learn
- Boyle's Law: the volume of a gas is inversely proportional to its pressure
- Charles' Law: the volume of a gas is directly proportional to its temperature
- Gay-Lussac's Law: the pressure of a gas increases when volume is held constant and heat is applied
- Avogadro's Law: equal volumes of gas at the same temperature and pressure contain the same number of gas particles
- Amontons' Law: the pressure of a gas is directly proportional to its temperature when volume is held constant

Boyle's Law: the volume of a gas is inversely proportional to its pressure
The volume of a gas is inversely proportional to its pressure. This is known as Boyle's Law, named after the Irish chemist Robert Boyle, who carried out experiments in the 17th century to determine the relationship between the pressure and volume of a gas.
Boyle's Law can be applied to understand the behaviour of gases in various situations, including in balloons. For example, as a weather balloon rises through the atmosphere to regions of lower pressure, the volume of the gas inside the balloon increases, causing the balloon to get larger. This is because the external pressure from the atmosphere decreases, so the gas inside the balloon expands until the internal and external pressures are equal.
Mathematically, Boyle's Law can be expressed as:
\[ PV = \text{constant} \]
Where P is pressure and V is volume. Dividing both sides by P gives:
\[ V = \frac{\text{constant}}{P} = \text{constant} \left( \frac{1}{P} \right) \]
This equation illustrates the inverse relationship between P and V, where the volume of a gas is proportional to the reciprocal of its pressure.
Boyle's experiments involved using a J-shaped tube partially filled with mercury. A small amount of gas was trapped above the mercury column, and its volume was measured at atmospheric pressure and a constant temperature. More mercury was then added to the open arm to increase the pressure on the gas sample, and the resulting volume was measured. This process was repeated to gather data on the relationship between pressure and volume.
Boyle's Law is one of several gas laws that describe the behaviour of gases, along with Charles' Law, Gay-Lussac's Law, and Avogadro's Law. These laws relate the pressure, volume, temperature, and amount of a gas, and were established by early scientists driven by a desire to understand nature and a quest to build balloons in which they could fly.
Kepler's Third Law: Beyond Our Sun's Reach?
You may want to see also

Charles' Law: the volume of a gas is directly proportional to its temperature
Charles's Law, named after the French scientist and balloon flight pioneer Jacques Alexandre César Charles, states that the volume of a given amount of gas is directly proportional to its temperature on the Kelvin scale when the pressure is held constant. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This relationship can be expressed mathematically as:
> [latex]V\propto T\qquad\text{or}\qquad{V}=\text{constant}\cdot T\qquad\text{or}\qquad{V}=k\cdot T\qquad\text{or}\qquad{V}_{1}\text{/}{T}_{1}={V}_{2}\text{/}{T}_{2} [/latex]
Where:
- V is the volume of the gas
- T is the temperature of the gas on the Kelvin scale
- K is a proportionality constant that depends on the amount and pressure of the gas
For example, if you have a balloon filled with air and you put it in the refrigerator, the gas inside gets colder and the balloon shrinks. If you then take the cold balloon out of the refrigerator and let it warm up, it will expand again as the gas inside increases in temperature.
Charles's Law can also be used to explain why hot air balloons rise. When heat is added to the air inside the balloon, the molecules inside move further away from each other, causing the air inside to expand. This reduces the density of the balloon, and when the density of the balloon is less than the density of the outside air, the balloon rises.
Charles's Law is one of several gas laws that describe how gases behave, along with Boyle's Law, Gay-Lussac's Law, and Avogadro's Law. These laws were established by early scientists who were driven by a desire to understand nature and a quest to build balloons in which they could fly.
Understanding the Scope of Fair Lending Laws
You may want to see also

Gay-Lussac's Law: the pressure of a gas increases when volume is held constant and heat is applied
Gay-Lussac's Law, also known as Amonton's Law, states that the pressure exerted by a gas is directly proportional to its absolute temperature when the volume and mass are held constant. This law was formulated by French chemist Joseph Gay-Lussac in the early 19th century, although Amonton proved the same law earlier by making a thermometer. Gay-Lussac's Law can be expressed mathematically as:
P ∝ T
Or
P = kT
Where P is the pressure exerted by the gas, T is the absolute temperature of the gas, and k is a constant. This law implies that the ratio of the initial pressure and temperature is equal to the ratio of the final pressure and temperature for a gas of a fixed mass kept at a constant volume, which can be expressed as:
P1/T1 = P2/T2 = k
This law has various real-life applications. For example, it can explain why a balloon rises when heat is added to the air inside it. It can also explain why a car's tire pressure increases after driving or why the pressure inside a pressurised aerosol can increases when left in the sun.
Gay-Lussac's Law is one of four gas laws, along with Boyle's Law, Charles's Law, and Avogadro's Law, that describe how gases behave. These individual laws were eventually combined into a single equation known as the ideal gas law, which relates gas quantities and is quite accurate for low pressures and moderate temperatures.
Laws for Humans: Do Animals and AI Obey, Too?
You may want to see also

Avogadro's Law: equal volumes of gas at the same temperature and pressure contain the same number of gas particles
The Italian scientist Amedeo Avogadro first advanced his hypothesis in 1811, which later became known as Avogadro's Law. This law states that equal volumes of all gases, when measured under the same conditions of temperature and pressure, contain the same number of molecules.
In other words, Avogadro's Law tells us that if you have two containers of the same volume, filled with different gases, but at the same temperature and pressure, then both containers will have the same number of gas molecules. This is true regardless of the type of gas or the size of the molecules.
Avogadro's Law is a specific case of the ideal gas law, which combines several other gas laws: Boyle's Law, Amontons's Law, Charles's Law, and Gay-Lussac's Law. These laws describe the relationships between the pressure, volume, temperature, and amount of gas present.
The mathematical equation for Avogadro's Law is:
V ∝ n
Or
V = k × n
Where V is the volume of the gas, n is the amount of substance (in moles), and k is a constant when temperature and pressure are fixed.
This law is useful for calculating the quantity of gas in a container and has practical applications, such as determining the pressure inside a car tyre or how a hot air balloon rises.
Hunting Laws on Private Property in North Carolina
You may want to see also

Amontons' Law: the pressure of a gas is directly proportional to its temperature when volume is held constant
Amontons' Law, a fundamental principle in the study of gases, establishes a direct relationship between the pressure and temperature of a gas when maintained at a constant volume. This principle is particularly applicable to understanding the behaviour of gases in confined spaces, such as balloons.
Amontons' Law states that as the temperature of a gas increases, so does its pressure, provided that the volume remains unchanged. This relationship is directly proportional, meaning that a linear correlation exists between pressure and temperature. If the temperature rises, the pressure follows suit, and vice versa, as long as the volume is held constant.
In the context of a balloon, this law helps explain the behaviour of the gas within it. When you inflate a balloon, you are introducing gas molecules into the confined space inside the balloon. These molecules are in constant motion, colliding with each other and the walls of the balloon. The energy of these collisions corresponds to the pressure exerted by the gas.
As you increase the temperature of the gas inside the balloon, the kinetic energy of the gas molecules also increases. They move faster and collide with greater force against the walls of the balloon, resulting in an increase in pressure. Conversely, decreasing the temperature reduces the kinetic energy of the molecules, leading to a decrease in pressure.
Amontons' Law provides a foundation for understanding and predicting the behaviour of gases in various situations, including those beyond the confines of a balloon. It is essential to consider this law when studying gases in sealed containers, engines, or atmospheric conditions, as it helps elucidate the influence of temperature changes on gas pressure in these contexts. By applying Amontons' Law, scientists and engineers can make informed predictions and design solutions for a multitude of applications involving gases.
Applying to Law School: 3 or 4 Applications Enough?
You may want to see also
Frequently asked questions
Boyle's Law states that the volume of a fixed amount of gas is inversely proportional to its pressure when the temperature is held constant. In other words, as the pressure on a gas increases, the volume decreases, and vice versa. This is why weather balloons get larger as they rise through the atmosphere to regions of lower pressure – the volume of the gas inside the balloon increases in response to the decrease in external pressure.
Charles' Law states that the volume of a given amount of gas is directly proportional to its temperature when the pressure is held constant. In other words, as the temperature of a gas increases, so does its volume, and vice versa. This is why hot air balloons rise – when heat is added to the air inside the balloon, the molecules move further apart, increasing the volume and decreasing the density of the balloon, causing it to ascend.
Gay-Lussac's Law states that if the volume of a gas is kept constant and heat is applied, the pressure of the gas will increase. This is because the gas molecules have more kinetic energy, causing them to hit the walls of their container with more force. This is why the air pressure inside car tyres increases when the car is driven – friction between the tyres and the road causes the air inside the tyres to heat up, and as the tyres are a fixed-volume container, the pressure increases.
Avogadro's Law states that the volume of a gas is directly proportional to the number of moles of gas present when temperature and pressure are held constant. In other words, as more gas is added to a fixed-volume container, the pressure inside the container will increase.