Charles's Law, a fundamental principle in thermodynamics, describes the relationship between the volume and temperature of a gas when pressure is held constant. Named after French scientist Jacques Charles, the law states that the volume of a given mass of gas is directly proportional to its absolute temperature. This law is expressed mathematically as $V \propto T$ or $V/T = k, where $V$ is volume, $T$ is temperature in Kelvin, and $k$ is a constant. It is crucial to use the Kelvin scale for temperature in Charles's Law because it is an absolute temperature scale, starting from absolute zero where molecular motion theoretically ceases.
What You'll Learn
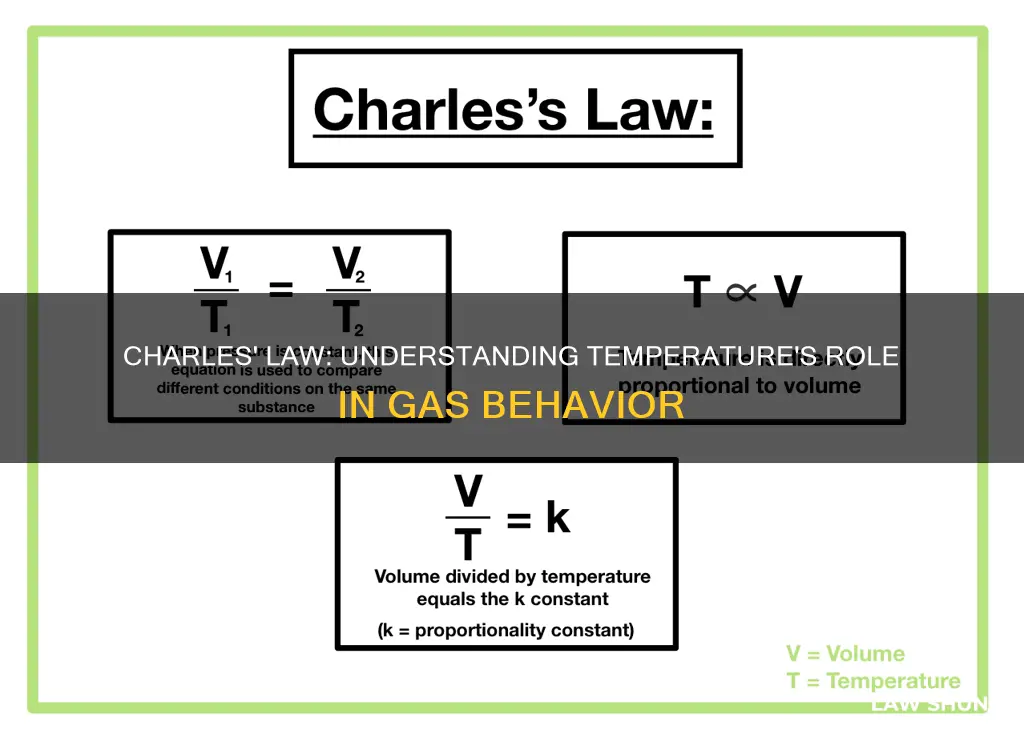
- Charles's Law is expressed mathematically as V ∝ T or V/T = k
- V is volume, T is temperature, and k is a constant
- The Kelvin scale must be used as zero on the Kelvin scale corresponds to a complete stoppage of molecular motion
- Charles's Law is a special case of the ideal gas law
- The law is named after French scientist Jacques Charles

Charles's Law is expressed mathematically as V ∝ T or V/T = k
Charles's Law, also known as the law of volumes, is an experimental gas law that describes the relationship between the volume and temperature of a gas when pressure remains constant. This law is expressed mathematically as V ∝ T or V/T = k, where V represents volume, T represents temperature, and k is a constant.
The mathematical expression V ∝ T indicates that the volume of a gas is directly proportional to its temperature in Kelvin. In other words, as the temperature of a gas increases, its volume also increases proportionally, assuming the pressure remains constant. Conversely, if the temperature decreases, the volume of the gas will decrease proportionally. This relationship can be observed in a balloon filled with gas; when the temperature increases, the gas inside the balloon expands, and when the temperature decreases, the gas contracts.
The alternative expression, V/T = k, further highlights the constant k, which represents the situation when the gas is at absolute zero temperature (0 Kelvin). At this point, the volume of the gas theoretically becomes zero. The value of k depends on the specific pressure and amount of gas being considered.
Charles's Law can also be written as V1/T1 = V2/T2, where V1 and V2 represent the initial and final volumes, while T1 and T2 represent the initial and final temperatures, respectively. This form of the equation allows for easy comparison of the same substance under two different sets of conditions.
The discovery of Charles's Law is credited to French scientist Jacques Charles, who formulated the original law in his unpublished work from the 1780s. However, it was later refined and popularised by Joseph Louis Gay-Lussac in the early 19th century. Charles's Law is a fundamental concept in physics and chemistry, with practical applications in various fields, including engineering, meteorology, and industrial processes.
Oregon Business Laws: What You Need to Know
You may want to see also

V is volume, T is temperature, and k is a constant
Charles's Law, also known as the law of volumes, is an experimental gas law that describes how gases expand when heated. The law was formulated by French physicist Jacques Charles in the 1780s.
The law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant. This relationship can be expressed as:
$$\frac{V}{T} = k$$
Or
$$V = kT$$
Where:
- $V$ is the volume of the gas
- $T$ is the temperature of the gas (measured in kelvins)
- $k$ is a constant for a particular pressure and amount of gas
This equation shows that the volume of a gas is directly proportional to its temperature when pressure is held constant. In other words, as the temperature of a gas increases, its volume will also increase proportionally, and conversely, a decrease in temperature will lead to a decrease in volume.
The Kelvin scale is used in Charles's Law because zero on this scale corresponds to a complete stoppage of molecular motion. The relationship between Celsius and Kelvin temperatures is given by:
$$\text{K} = \: ^\text{o} \text{C} + 273$$
Charles's Law can be used to compare changing conditions for a gas. If $V_1$ and $T_1$ represent the initial volume and temperature of a gas, and $V_2$ and $T_2$ represent the final volume and temperature, the equation becomes:
$$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
This equation can be used to calculate any of the four variables as long as the other three are known. It's important to note that the temperatures must be expressed in Kelvin for this equation to hold true.
For example, let's say we have a balloon filled to a volume of $2.20 \: \text{L}$ at a temperature of $22^\text{o} \text{C}. If we then heat the balloon to a temperature of $71^\text{o} \text{C}, what will be the new volume?
First, we convert the temperatures to Kelvin:
$$T_1 = 22^\text{o} \text{C} = 295 \: \text{K}$$
$$T_2 = 71^\text{o} \text{C} = 344 \: \text{K}$$
Now, we can use Charles's Law to solve for the unknown volume, $V_2$:
$$V_2 = \frac{V_1 \times T_2}{T_1}$$
Substituting the known values:
$$V_2 = \frac{2.20 \: \text{L} \times 344 \: \text{K}}{295 \: \text{K}} \approx 2.57 \: \text{L}$$
So, the volume of the balloon increases to approximately $2.57 \: \text{L}$ as the temperature rises.
Benford's Law: A Strategy to Win at Roulette?
You may want to see also

The Kelvin scale must be used as zero on the Kelvin scale corresponds to a complete stoppage of molecular motion
Charles's Law, a fundamental principle in thermodynamics, describes the relationship between the volume and temperature of a gas held at constant pressure. While the law itself doesn't explicitly require the use of the Kelvin scale, it is often employed when working with gas laws due to its absolute temperature measurement.
The Kelvin scale is an absolute temperature scale, starting from absolute zero, the point at which molecular motion theoretically ceases. This is an important consideration when applying Charles's Law, as the law assumes that gas molecules have negligible volume and no intermolecular forces. At absolute zero, a gas would have zero energy, and its molecules would be completely stationary, causing it to exist in a solid state rather than a gaseous state. Therefore, the Kelvin scale is necessary to ensure that temperatures used in Charles's Law are within the range where gases will exist.
Additionally, the Kelvin scale simplifies mathematical relationships in gas law calculations. The temperature in Kelvin is directly proportional to the average kinetic energy of gas particles. This makes it a preferred scale for gas law calculations as it avoids negative temperatures and ensures consistency in calculations. Using Celsius or Fahrenheit scales can result in negative temperatures, leading to undefined or unrealistic volume values.
The Kelvin scale also allows for easier comparisons of the heat energy of gases. For example, a gas at 10 Kelvin has only half the heat energy of a gas at 20 Kelvin. This relationship does not hold true when comparing temperatures in Celsius or Fahrenheit.
In summary, the Kelvin scale is essential when applying Charles's Law because zero on the Kelvin scale corresponds to a complete stoppage of molecular motion, ensuring that temperatures used in the law are within the range where gases exist. The Kelvin scale also simplifies calculations, avoids negative temperatures, and allows for straightforward comparisons of heat energy between gases.
Gay-Lussac's Law: Everyday Applications of Gas Pressure
You may want to see also

Charles's Law is a special case of the ideal gas law
Charles's Law, also known as the Law of Volumes, is a fundamental principle in the field of thermodynamics. It is named after the French scientist Jacques Charles, who formulated the original law in his unpublished work in the 1780s. This law describes the relationship between the volume and temperature of a gas when pressure remains constant.
Charles's Law can be stated as: the volume of a gas is directly proportional to its absolute temperature when pressure remains constant. This relationship can be expressed mathematically as V ∝ T or V/T = k, where V is volume, T is temperature in Kelvin, and k is a constant. This implies that as the temperature of a gas increases, its volume will also increase proportionally, and vice versa.
The ideal gas law incorporates specific gas constants that account for the molecular weight of the gas. While Charles's Law provides a useful framework, it's important to consider the characteristics of the gas in question. Real gases may exhibit deviations from ideal behaviour, especially under extreme conditions.
In summary, Charles's Law is a foundational concept in physics and chemistry, with practical applications in various fields, including engineering, meteorology, and industrial processes. Its special case status within the ideal gas law highlights its relevance in understanding the behaviour of gases under specific conditions.
Detaching with Purpose: Applying the Law of Detachment
You may want to see also

The law is named after French scientist Jacques Charles
Charles's Law, a fundamental principle in the field of thermodynamics, is named after French scientist Jacques Charles. Born in Beaugency, France, on November 12, 1746, Jacques Charles was a mathematician, physicist, and inventor. He made significant contributions to the understanding of gas behaviour through his experiments in the late 18th century.
Charles's Law describes the relationship between the volume and temperature of an ideal gas when pressure remains constant. It states that the volume of a gas is directly proportional to its absolute temperature when pressure is held constant. This law can be expressed mathematically as V ∝ T or V/T = k, where V is volume, T is temperature, and k is a constant.
The origins of Charles's Law can be traced back to Jacques Charles's experiments on the thermal expansion of gases in 1787. He filled five balloons to the same volume with different gases and raised the temperature to 80°C. Charles observed that the volume of each balloon increased by the same amount, regardless of the type of gas. This discovery led to the formulation of Charles's Law, which was later refined and popularised by Joseph Louis Gay-Lussac in the early 19th century.
Jacques Charles was also a pioneer in ballooning. Along with the Robert brothers, he constructed and flew the world's first hydrogen-filled gas balloon in 1783. He was the first to ascend in a hydrogen balloon, reaching an altitude of about 1,800 feet. Charles's contributions to science extended beyond gas laws and ballooning, as he also developed several inventions, including a hydrometer and a reflecting goniometer. He was elected to the Académie des Sciences in 1795 and became a professor of physics, sharing his knowledge with students.
Kepler's Third Law: Moons Included?
You may want to see also
Frequently asked questions
The Kelvin scale is an absolute temperature scale, starting from absolute zero where molecular motion theoretically ceases. This aligns with the principles of Charles' Law, which states that the volume of a gas is directly proportional to its absolute temperature. Using Kelvin simplifies calculations and ensures consistency by avoiding negative temperatures.
According to Charles' Law, as the temperature of a gas decreases, so does its volume. At -273°C, the volume of a gas would theoretically be zero as this is the point of absolute zero where molecular motion stops. However, gases would condense into a liquid state before reaching this temperature.
Charles' Law primarily applies to ideal gases, which assume negligible molecular volume and no intermolecular forces. Real gases deviate from these ideal conditions, especially at high pressures and low temperatures, and may not follow Charles' Law precisely. However, it is still a valuable framework for understanding gas behaviour.







