Raoult's law, established in 1887, is a law of thermodynamics that states that the vapour pressure of a solution decreases when substances are mixed. It is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. However, it is only valid for ideal solutions, which are rare. In the case of immiscible liquids, the vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently. Therefore, there is no lowering of vapour pressure, and Raoult's law does not hold true for immiscible solutions.
What You'll Learn

Raoult's Law and Partial Vapour Pressure
Raoult's Law, a principle of physical chemistry, was established by French chemist François-Marie Raoult in 1887. It states that the partial vapour pressure of each component in an ideal mixture of liquids is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture. In other words, the vapour pressure of a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, Raoult's law can be written as:
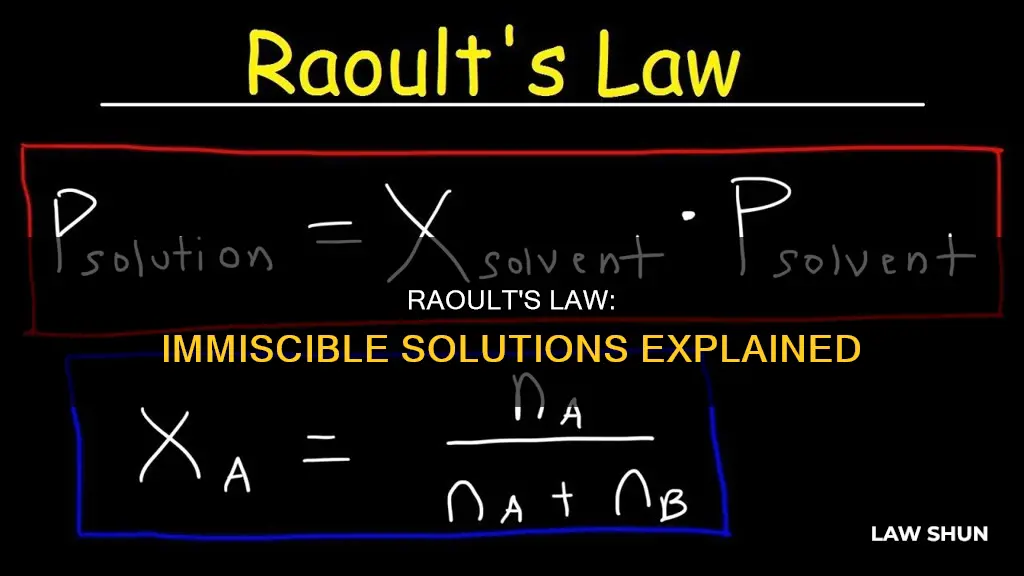
> Psolution = ΧsolventP0solvent
Where:
- Psolution = vapour pressure of the solution
- Χsolvent = mole fraction of the solvent
- P0solvent = vapour pressure of the pure solvent
Raoult's law is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. It assumes that intermolecular forces between different molecules and similar molecules are equal. This is analogous to the ideal gas law, which assumes that intermolecular forces between dissimilar molecules are zero or non-existent.
Raoult's law is applied to solutions, whereas the ideal gas law is applied to gases. Raoult's law can also be applied to non-ideal solutions by considering the interactions between molecules of different substances.
Raoult's law is particularly useful in calculating the molecular mass of an unknown solute. It also helps us understand the behaviour of solutions and the effects of solutes on solvent vapour pressure.
However, it is important to note that Raoult's law is only valid for ideal solutions, which are rare. Most solutions deviate from Raoult's law due to differences in attractive forces between the components. When the adhesion between dissimilar molecules is stronger than the cohesion between similar molecules, the vapour pressure decreases, resulting in a negative deviation from Raoult's law. Conversely, when adhesion is weaker than cohesion, the vapour pressure increases, leading to a positive deviation.
Ohm's Law in RLC Circuits: Understanding the Application
You may want to see also

Raoult's Law and Vapour Pressure of Pure Solvent
Raoult's Law, a principle of physical chemistry, was established by French chemist François-Marie Raoult in 1887. It states that the vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. The law is represented by the following equation:
Psolution = ΧsolventP0solvent
Where:
- Psolution is the vapour pressure of the solution
- Χsolvent is the mole fraction of the solvent
- P0solvent is the vapour pressure of the pure solvent
Raoult's Law is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. It is important to note that Raoult's Law only applies to ideal solutions, where the solvent-solute interaction is the same as the solvent-solvent or solute-solute interaction. In reality, such solutions are rare.
When a solute is added to a solvent, the solute molecules occupy the space between the solvent molecules on the surface of the solution. This results in a decrease in the number of solvent molecules that can escape into the vapour phase, leading to a lower vapour pressure.
Raoult's Law can also be applied to non-ideal solutions by incorporating factors that account for the interactions between molecules of different substances. The fugacity coefficient and the activity coefficient are used as correction factors for gas non-ideality and interactions in the liquid phase, respectively.
Understanding Minimum Wage Laws for 1099 Contractors
You may want to see also

Raoult's Law and Mole Fraction
Raoult's Law, established in 1887, is named after French chemist François-Marie Raoult. It states that the vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution.
Mathematically, Raoult's Law can be written as:
> Psolution = ΧsolventP0solvent
Where:
- Psolution = vapour pressure of the solution
- Χsolvent = mole fraction of the solvent
- P0solvent = vapour pressure of the pure solvent
Raoult's Law can be used to calculate the molecular mass of an unknown solute. It is also similar to the ideal gas law, except that it applies to solutions.
Raoult's Law only works for ideal solutions, which are rare. It assumes that the intermolecular forces between different molecules and similar molecules are equal. In reality, many liquids in a mixture do not have the same uniformity in terms of attractive forces, and so these solutions tend to deviate from the law.
Raoult's Law can be applied to non-ideal solutions by incorporating factors that consider the interactions between molecules of different substances.
The mole fraction is calculated as follows:
> Χi = moles of component i in the solution / total moles of all components in the solution
Raoult's Law can be used to determine the partial pressure of each component in an ideal mixture of liquids. The partial pressure of a component is equal to the vapour pressure of the pure component multiplied by its mole fraction in the mixture.
> Pi = Pi*xi
Where:
- Pi = partial pressure of component i
- Pi = equilibrium vapour pressure of the pure component i
- Xi = mole fraction of component i in the liquid or solid solution
The total vapour pressure of a solution can be determined by combining Raoult's Law with Dalton's Law of partial pressures:
> Ptot = PAsolventΧAsolvent + PBsolventΧBsolvent
Where:
- Ptot = total vapour pressure of the solution
- PA solvent and ΧAsolvent = partial vapour pressure and mole fraction of solvent A
- PB solvent and ΧBsolvent = partial vapour pressure and mole fraction of solvent B
Raoult's Law can be used to calculate the vapour pressure of a solution when a non-volatile solute is dissolved into a solvent to form an ideal solution. The vapour pressure of the solution will be lower than that of the pure solvent, and this decrease is directly proportional to the mole fraction of the solute.
Benford's Law: Can It Verify Voting Results?
You may want to see also

Raoult's Law and Total Vapour Pressure
Raoult's Law, named after French chemist François-Marie Raoult, is a principle of physical chemistry with applications in thermodynamics. It states that the vapour pressure of a solvent in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. In other words, it predicts that when a substance is dissolved in a solution, the vapour pressure of the solution will decrease.
Mathematically, Raoult's Law can be written as:
> Psolution = ΧsolventP0solvent
Where:
- Psolution = vapour pressure of the solution
- Χsolvent = mole fraction of the solvent
- P0solvent = vapour pressure of the pure solvent
Raoult's Law is only applicable to ideal solutions, where the solvent-solute interaction is the same as the solvent-solvent or solute-solute interaction. In reality, ideal solutions are rare, and most solutions deviate from Raoult's Law to some degree.
Raoult's Law can be used to calculate the molecular mass of an unknown solute. It is also similar to the ideal gas law, assuming that intermolecular forces between different molecules are equal.
When considering the total vapour pressure of a solution, Raoult's Law can be combined with Dalton's Law of partial pressures to give:
> p=p°AxA+p°Bx°B+...
Where:
- P = total vapour pressure of the solution
- P°A and p°B = vapour pressures of pure components A and B
- XA and xB = mole fractions of components A and B in the liquid or solid solution
This equation demonstrates that the vapour pressure of the solution is the mole-weighted mean of the individual vapour pressures.
In the case of immiscible liquids, Raoult's Law does not hold true. In such mixtures, the vapour pressure of the solution increases and is equal to the vapour pressures of the two solvents independently. Therefore, there is no lowering of vapour pressure in the solution, contrary to what Raoult's Law predicts.
Usury Laws and Business Loans in Texas: What's the Verdict?
You may want to see also

Raoult's Law and Ideal Solutions
Raoult's law, established in 1887, is a law of thermodynamics that relates to physical chemistry. It was named after François-Marie Raoult, a French chemist who discovered that when substances were mixed in a solution, the vapour pressure of the solution decreased simultaneously.
Raoult's law states that a solvent's partial vapour pressure in a solution (or mixture) is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. This can be expressed mathematically as:
- Psolution = ΧsolventP0solvent
- Psolution = vapour pressure of the solution
- Χsolvent = mole fraction of the solvent
- P0solvent = vapour pressure of the pure solvent
Raoult's law is used to calculate the relative lowering of vapour pressure of a solvent when a solute is added. In the case of a non-volatile solute in a volatile solvent mixture, the vapour pressure of the solution decreases.
Raoult's law is considered valid for ideal solutions, which are solutions where the solvent-solute interaction is the same as the solvent-solvent or solute-solute interaction. In other words, both the solute and the solvent require the same amount of energy to escape to the vapour phase as they would in their pure states.
Ideal solutions are generally rare and hard to find, as they require the chemical components to be chemically identical. These solutions follow Raoult's law at almost all levels of concentration and temperature. They are characterised by:
- A zero enthalpy of mixing, meaning no heat is released or absorbed during the mixing of two pure components.
- A zero volume of mixing, meaning there is no contraction or expansion during the mixing of two components.
- Similar solute-solute, solvent-solvent, and solute-solvent interactions.
Some examples of ideal solutions include:
- N-hexane and n-heptane
- Bromoethane and chloroethane
- Chlorobenzene and bromobenzene
- Ethyl bromide and ethyl iodide
- Toluene and benzene
Non-ideal solutions, on the other hand, deviate from Raoult's law and can be further classified into two types:
- Positive deviation from Raoult's law: The vapour pressure of the component is greater than expected, and the solute-solvent forces of attraction are weaker than the solute-solute and solvent-solvent interactions.
- Negative deviation from Raoult's law: The vapour pressure of the component is less than expected, and the solute-solvent forces of attraction are stronger than the solute-solute and solvent-solvent interactions.
Carry Laws: Private Property Exempt?
You may want to see also
Frequently asked questions
No, Raoult's Law is only applicable to ideal solutions, which are rarely found. Immiscible solutions do not have the same uniformity in terms of attractive forces, and therefore deviate from the law.
Raoult's Law states that a solvent's partial vapour pressure in a solution is equal to the vapour pressure of the pure solvent multiplied by its mole fraction in the solution. It was established in 1887 by French chemist François-Marie Raoult.
Raoult's Law can be used to calculate the molecular mass of an unknown solute. It is also used as a basis for the process of distillation, which is a common method for separating mixtures.