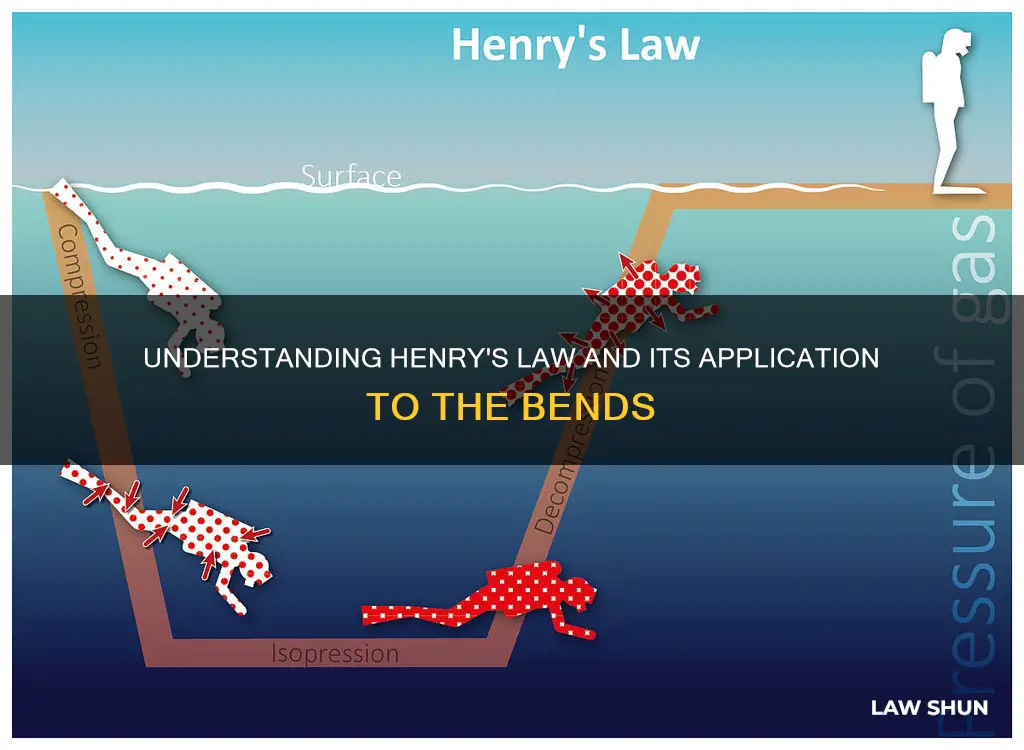
Decompression sickness, also known as the bends, is a medical condition caused by dissolved gases emerging from solution as bubbles inside the body tissues during decompression. This occurs when a diver ascends to the surface of the ocean too rapidly, causing nitrogen gas to be rapidly released from the bloodstream. As divers descend into the ocean, the external pressure on their bodies increases, necessitating an increase in the pressure of the air they breathe to prevent their chests and lungs from collapsing. According to Henry's Law, as the pressure increases, the solubility of gases such as nitrogen and helium in the blood also increases. When divers ascend too quickly, the rapid decrease in pressure can lead to the formation of nitrogen bubbles, resulting in the bends. This condition causes pain and can lead to injuries involving the nervous system.
| Characteristics | Values |
|---|---|
| What is it? | A painful and dangerous condition called "the bends" |
| What causes it? | The rapid release of nitrogen gas from the bloodstream |
| How does it happen? | Divers' bodies are exposed to increased pressure underwater, which, according to Henry's Law, increases the solubility of gases like nitrogen in the bloodstream. When a diver ascends too quickly, the pressure decreases rapidly, causing nitrogen bubbles to form in the blood and tissues. |
| How to prevent it? | Divers should ascend slowly to the surface, allowing for a gradual release of nitrogen and reducing the impact of pain. |
| Alternative prevention methods | Spending time in a decompression chamber; breathing a helium-oxygen mixture instead of air; using a dry suit. |
| Symptoms | Coughing and chest pain; dizziness and paralysis; joint pain; blisters under the skin. |
What You'll Learn

Pressure and solubility
Mathematically, Henry's Law can be expressed as:
C = kP
Where:
- C is the concentration of the dissolved gas at equilibrium
- P is the partial pressure of the gas
- K is the Henry's Law constant, which varies depending on the gas, solvent, and temperature
The application of Henry's Law is particularly relevant in deep-sea diving. As a diver ventures deeper underwater, the pressure in their bloodstream increases. According to Henry's Law, this increase in pressure leads to a higher solubility of gases, including nitrogen, in the bloodstream. If a diver ascends too quickly, the rapid decrease in pressure can cause nitrogen bubbles to form in the blood. This condition is known as "the bends" and can result in pain, nerve damage, and even death.
The solubility of gases in liquids is also influenced by temperature. Generally, increasing the temperature leads to a decrease in the solubility of gases. This is because higher temperatures provide the thermal energy needed to overcome the attractive forces between the gas and solvent molecules. However, the relationship between temperature and solubility is complex and varies depending on the specific gas and solvent involved.
The influence of temperature on gas solubility is described by the equation:
P = Hv × M or P = M / Hv
Where:
- P is the pressure
- Hv is Henry's Proportionality Constant
- M is the molar concentration of the gas
In summary, pressure and solubility are interconnected, especially for gases in liquids. Henry's Law explains how an increase in pressure leads to greater solubility, while the inverse relationship between temperature and solubility is also important to consider. These principles have practical applications, such as in deep-sea diving, where understanding and managing pressure and solubility are crucial for safety.
Franchisees and Antitrust Laws: What's the Verdict?
You may want to see also

Gas release from blood
Decompression sickness, also known as the bends, is a medical condition that occurs when dissolved gases are released from the blood and form bubbles inside the body's tissues. This happens when there is a rapid decrease in pressure, such as when a scuba diver ascends too quickly from a deep dive.
Henry's law states that the amount of gas that can be dissolved in a liquid is directly proportional to the partial pressure of the gas above the liquid. In the context of scuba diving, this means that as the pressure increases with depth, more nitrogen (an inert gas) is dissolved into the bloodstream. This is because the air we breathe is composed of 21% oxygen, 78% nitrogen, and less than 1% other gases.
Gas Release During Ascent
When a diver ascends, the surrounding pressure decreases, and the nitrogen that was dissolved in the blood at higher pressure begins to come out of solution. This leads to the formation of nitrogen bubbles in the bloodstream, which can cause blockages in capillaries and veins, as well as compress or rupture tissues. The bubbles can also induce endothelial damage and trigger inflammatory responses in the body.
Symptoms and Treatment
The bends can cause a range of symptoms, from joint pain and skin rashes to paralysis and even death. Treatment for decompression sickness typically involves recompression therapy, where the diver is placed in a hyperbaric oxygen chamber to gradually reduce the pressure and allow the body to safely eliminate the excess nitrogen. Divers are also taught to ascend slowly and make decompression stops to minimise the risk of developing the bends.
Kepler's Law of Areas: Hyperbola Application Explored
You may want to see also

Formation of nitrogen bubbles
The Bends, or decompression sickness, is caused by the formation of nitrogen bubbles in the blood and body tissues. This occurs when a scuba diver ascends too quickly, resulting in a rapid decrease in pressure. As the pressure in the diver's surroundings decreases, the nitrogen dissolved in their bloodstream and body tissues escapes and forms bubbles. This process is described by Henry's Law, which states that the solubility of a gas in a liquid is proportional to the pressure of the gas.
During a dive, as a diver descends, the pressure increases, and more nitrogen from the air they are breathing dissolves into their bloodstream. This is in accordance with Henry's Law, which states that as pressure increases, the solubility of a gas in a liquid also increases. However, when a diver ascends too quickly, the pressure decreases rapidly, and the nitrogen is unable to escape the body slowly through exhalation. Instead, it comes out of solution rapidly, forming bubbles in the blood and body tissues. These bubbles can form anywhere in the body but often settle in the major joints, causing intense pain and the characteristic bending over that gives the condition its name.
The risk of developing decompression sickness is influenced by several factors, including the rate of ascent, the duration of the dive, and the interval between dives. A faster ascent rate, longer dive duration, and shorter interval between dives increase the risk of DCS. Additionally, exertion during the deepest phase of a dive and exertion immediately following a dive can stimulate bubble formation and increase the likelihood of bubbles passing through the lungs and entering the bloodstream. Individual factors such as body temperature, body fat content, dehydration, and previous injuries can also contribute to the risk of developing DCS.
To prevent the formation of nitrogen bubbles and reduce the risk of decompression sickness, divers should follow recommended ascent rates, typically around 10 metres (33 feet) per minute, and adhere to decompression schedules or use dive computers to monitor their ascent speed and limit their exposure to pressure changes.
Hong Kong's Legal System: British Influence and Legacy
You may want to see also

Blocked capillaries
Lifestyle choices that can lead to blocked capillaries include standing or sitting for long periods of time, which can cause poor circulation in the legs and limbs. This, in turn, can cause the valves that regulate blood flow to become blocked by fat tissue. Obesity can also cause blocked capillaries in the legs, as the increased pressure on the body can interfere with circulation, straining the fragile lining of the veins and eventually causing the valves to give way, resulting in blood pooling.
Medical conditions that can cause blocked capillaries include blood clots, inflammation in the veins, constipation, rosacea, and systematic skin infections. Additionally, physical trauma, such as a bruise or injury, can permanently damage the tiny blood vessels in the skin, leading to blocked capillaries. This is more likely to occur in individuals with sensitive skin.
Ageing is another common cause of blocked capillaries, as our skin becomes thinner and weaker over time, making it more prone to trauma. The ageing process also reduces the strength of elastin fibres, leading to more extreme widenings near areas prone to wrinkles.
Black's Law Dictionary: Its Relevance to US Law Explained
You may want to see also

Decompression sickness
According to Henry's Law, the quantity of gas dissolved in a liquid is directly proportional to the partial pressure of the gas in equilibrium with the liquid. Therefore, as the pressure increases, the solubility of gases in the bloodstream also increases. When divers descend, the external pressure on their bodies increases, and they breathe pressurised air to balance this. As a result, gases like nitrogen and helium become increasingly soluble in the blood.
When divers ascend, the surrounding pressure decreases, and bubbles may form. These bubbles can cause symptoms by blocking blood vessels, rupturing or compressing tissue, and inducing endothelial damage. They can also travel via the blood to distant organs (arterial gas embolism). The bends refer to local joint or muscle pain due to decompression sickness but is often used as a synonym for any component of the disorder.
The risk of DCS can be managed through proper decompression procedures, and the condition has become uncommon. Divers use decompression schedules or dive computers to limit their exposure and monitor their ascent speed. If DCS is suspected, it is treated by hyperbaric oxygen therapy in a recompression chamber.
The symptoms of DCS vary and can range from joint pain and rashes to paralysis and death. They typically occur within an hour of surfacing in about 50% of patients and by six hours in 90%. In rare cases, symptoms can manifest up to 48 hours after surfacing, particularly after exposure to altitude following diving.
Cell Phone Laws: Private Property Exempt?
You may want to see also
Frequently asked questions
The bends, or decompression sickness, is a painful and potentially fatal condition that occurs when a diver ascends to the surface too quickly. It is caused by the rapid formation of nitrogen bubbles in the blood and other tissues, which can damage nerves, blood and lymphatic vessels, and cause clots.
Henry's Law states that the solubility of a gas in a liquid is directly proportional to the pressure of that gas. As a diver descends, the pressure increases, and their bloodstream absorbs more nitrogen. If the diver ascends too quickly, the pressure decreases rapidly, and the nitrogen is released too fast, forming bubbles in the blood.
Ascending slowly allows the excess nitrogen to be released gradually, reducing the impact on the diver. This helps to prevent the formation of bubbles and the resulting pain and other symptoms.
Helium is less soluble in the bloodstream than nitrogen, so it does not build up as much during a dive. This means that fewer bubbles form when the diver ascends, reducing the risk of the bends.