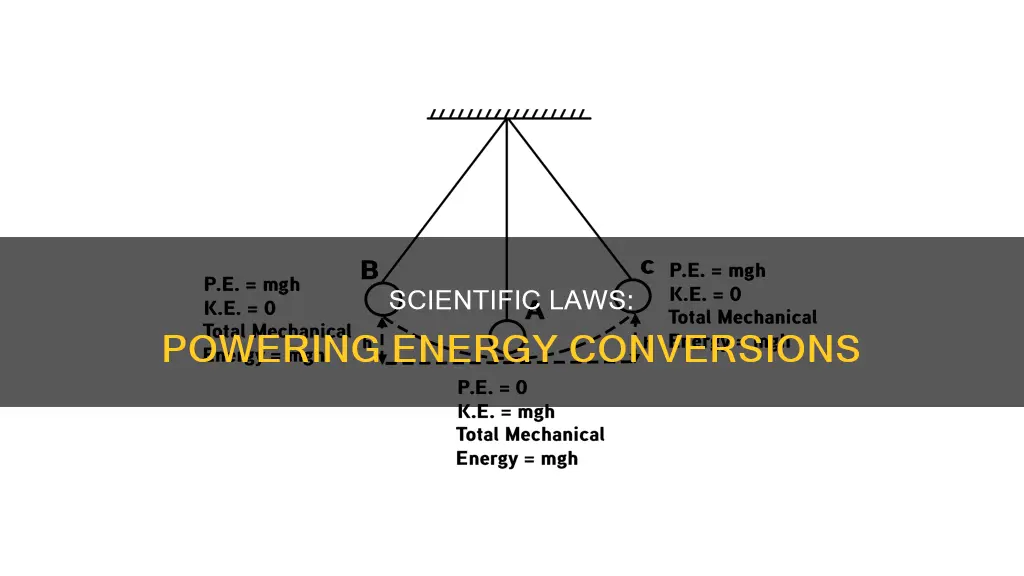
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. The law of conservation of energy states that energy can neither be created nor destroyed, only converted from one form to another. This means that the total amount of energy in a system remains constant unless it is an open system, in which case energy can enter or leave. This law is also known as the first law of thermodynamics.
Energy conversion occurs everywhere and every minute of the day. There are numerous forms of energy, including thermal, electrical, nuclear, electromagnetic, mechanical, chemical, and sound energy. The conversion of energy is essential to modern life, as it allows us to harness natural forms of energy and convert them into forms that can be used by humans. For example, a car engine burns gasoline, converting the chemical energy in the fuel into mechanical energy.
The efficiency of energy conversion varies depending on the forms of energy involved. Conversions to and from thermal energy, for instance, are often less efficient due to the disordered nature of thermal energy. On the other hand, conversions between non-thermal forms of energy, such as potential and kinetic energy, can occur with fairly high efficiency.
The study of how energy gets converted from one form to another is called thermodynamics, and it provides important insights into the scientific laws that govern energy conversions.
| Characteristics | Values |
|---|---|
| Definition of energy conversion | The process of changing one form of energy into another |
| Other names | Energy transformation |
| Law of energy conversion | The law of conservation of energy |
| First law of thermodynamics | Energy can neither be created nor destroyed, it can only be transformed from one form to another |
| Other names for the first law of thermodynamics | Law of energy conversion, Law of conservation of energy |
| Total amount of energy in the universe | Stays the same |
| Energy transformations in machines | Coal-fired power plants, conventional automobiles, electric generators, batteries, etc. |
What You'll Learn

The law of conservation of energy
The total energy within an isolated system remains constant over time, and any change in energy within a closed system is due to energy entering or leaving the system. This principle can be expressed mathematically:
> UT = Ui + W + Q
Where UT is the total energy of a system, Ui is the initial energy of a system, Q is the heat added or removed from the system, and W is the work done by or on the system.
The change in internal energy of a system can also be calculated using the equation:
> ΔU = W + Q
The concept of energy conservation is not limited to theoretical discussions; it has practical implications for energy conservation and sustainability. By reducing our demand on limited energy sources and exploring alternative energy options, we can work towards rebuilding and conserving our energy resources.
Zoning Laws and Private Roads: Who's in Control?
You may want to see also

Energy conversion in machines
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. Energy conversion occurs everywhere, every minute of the day. There are various types and forms of energy, including thermal energy, electrical energy, nuclear energy, electromagnetic energy, mechanical energy, chemical energy, and sound energy. The total amount of energy in a system remains the same, and this is known as the law of conservation of energy.
Another example of energy conversion in machines is a conventional automobile. In an automobile, the chemical energy in the fuel is converted into kinetic energy through combustion. This kinetic energy of the expanding gas is then transformed into linear piston movement. The linear piston movement further gets converted into rotary crankshaft movement, which eventually results in the linear motion of the automobile.
Energy conversion efficiency (η) is the ratio between the useful output of an energy conversion machine and the input in energy terms. The input and output can be in various forms, such as chemical, electric power, mechanical work, light (radiation), or heat. The resulting value, η (eta), ranges between 0 and 1, indicating that efficiencies cannot exceed 100%. This is because a value above 100% would result in a perpetual motion machine, which is impossible.
In recent years, there has been a growing interest in direct energy conversion devices, such as solar cells and fuel cells. These devices bypass the intermediate step of converting heat energy into electrical power, making them more efficient and environmentally friendly.
Equality Law: Sole Trader's Rights and Responsibilities
You may want to see also

Energy conversion in nature
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. This process occurs everywhere, every minute of the day.
The law of conservation of energy states that energy can neither be created nor destroyed, only transformed or transferred from one form to another. This is a fundamental law that has been observed to hold for all natural phenomena. The total amount of energy in an isolated system remains constant over time.
- Photosynthesis in plants: Solar energy is converted into chemical energy.
- Geothermal power plants: Heat energy is converted into electrical energy.
- Hydroelectric dams: Gravitational potential energy is converted into electrical energy.
- Wind energy: Converted into mechanical energy or electrical energy.
- Ocean Thermal Energy Conversion (OTEC): Heat energy is converted into electrical or mechanical energy.
These are just a few examples of how energy conversion occurs in nature, showcasing the transformation of energy from forms provided by nature into forms that can be used by humans.
Usury Laws and Car Loans: What You Need to Know
You may want to see also

Limitations of thermal energy conversion
Energy conversion, also known as energy transformation, is the process of changing one form of energy into another. There are many forms of energy, including thermal energy, electrical energy, nuclear energy, electromagnetic energy, mechanical energy, chemical energy, and sound energy.
Thermal energy is unique in that it cannot, in most cases, be converted into other forms of energy. Only a difference in the density of thermal/heat energy (temperature) can be used to perform work, and the efficiency of this conversion is much less than 100%. This is because thermal energy is a particularly disordered form of energy. It is spread out randomly among many available states of a collection of microscopic particles constituting the system. The measure of this disorder or randomness is entropy, and its defining feature is that the entropy of an isolated system never decreases.
The second law of thermodynamics states that the entropy of a closed system can never decrease. For this reason, thermal energy in a system may be converted to other kinds of energy with efficiencies approaching 100% only if the entropy of the universe is increased by other means. Otherwise, only a part of that thermal energy may be converted to other kinds of energy (and thus useful work). This is because the remainder of the heat must be reserved to be transferred to a thermal reservoir at a lower temperature. The increase in entropy for this process is greater than the decrease in entropy associated with the transformation of the rest of the heat into other types of energy.
To improve the efficiency of energy transformation, it is desirable to avoid thermal conversion. For example, the efficiency of nuclear reactors, where the kinetic energy of the nuclei is first converted to thermal energy and then to electrical energy, is around 35%. By directly converting kinetic energy to electric energy, the efficiency of the energy transformation process can be dramatically improved.
Romeo and Juliet Law: Massachusetts' Exception
You may want to see also

Direct energy conversion
Thermodynamics, the study of energy conversion, provides insights into how energy transformations occur. For example, in a coal-fired power plant, chemical energy in coal is converted into thermal energy through combustion. This thermal energy is then transformed into steam's thermal energy using a heat exchanger. The turbine then converts this thermal energy into mechanical energy, which is finally converted into electrical energy by a generator.
Overall, direct energy conversion is a vital aspect of energy transformation, offering simplified and efficient methods for converting different forms of energy to meet our energy needs.
Dalton's Law: Understanding External and Internal Respiration
You may want to see also
Frequently asked questions
The law of conservation of energy states that energy cannot be created or destroyed, only transformed from one form to another. The total amount of energy in a system remains constant unless energy is added or removed.
In a car engine, the chemical energy in gasoline is converted into mechanical energy. This is an example of how energy conversion allows us to use energy from natural processes to perform work.
The first law of thermodynamics states that energy can be transformed from one form to another but cannot be created or destroyed. This law is fundamental to understanding energy conversion and the various forms of energy, such as thermal, electrical, and mechanical energy.
Photosynthesis in plants is an excellent example of energy conversion in nature. Through photosynthesis, plants convert solar energy (electromagnetic radiation) into chemical energy, which is then released as heat and light during combustion. Another example is a waterfall, where potential energy is converted into kinetic energy.