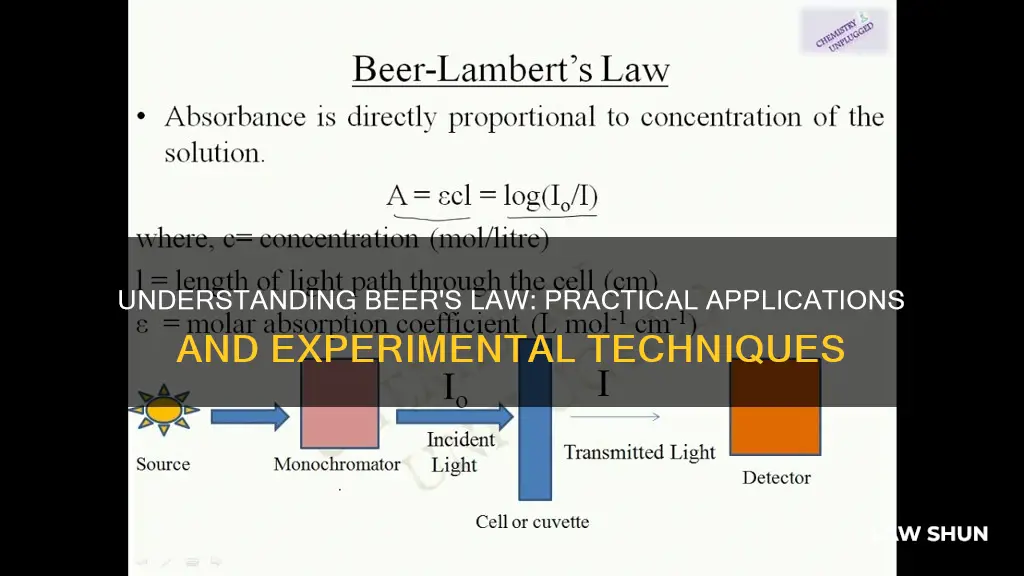
Beer's Law, also known as the Beer-Lambert Law, is an equation that relates light attenuation to the properties of the material through which the light is travelling. It is often used in UV-visible absorption spectroscopy. The law states that a chemical's concentration is directly proportional to a solution's absorbance. In other words, the more concentrated a solution is, the more light it absorbs. This is because a light beam becomes weaker as it passes through a chemical solution, due to the distance travelled through the solution and the increasing concentration.
The Beer-Lambert Law is expressed as:
> A = εlc
where:
- A is absorbance (no units)
- ε is the molar absorptivity with units of L mol-1 cm-1 (formerly called the extinction coefficient)
- l is the path length of the sample, usually expressed in cm
- c is the concentration of the compound in solution, expressed in mol L-1
There are three methods for applying Beer's Law, depending on the information available: using proportionality, graphing, and the Beer's Law equation.
What You'll Learn
- Beer's Law states that the concentration of a sample is directly proportional to its absorbance
- The law is used to find the solution concentration and assess oxidation
- The law is also used to measure the amount of bilirubin in blood samples
- Beer's Law is used in spectroscopy to describe the attenuation of solar radiation in the Earth's atmosphere
- The law is used in physics to describe the attenuation of particle beams

Beer's Law states that the concentration of a sample is directly proportional to its absorbance
Beer's Law, also known as the Beer-Lambert Law, states that the concentration of a sample is directly proportional to its absorbance. This means that as the concentration of a substance increases, so does the amount of light it absorbs.
The law is expressed as:
> A = εLc
Where:
- A is the amount of light absorbed by the sample at a specific wavelength
- Ε (epsilon) is the extinction coefficient at the molar level
- L is the length of time that light travels through the solution
- C is the concentration of the absorbing species
This equation can be used to determine the concentration of a chemical species in a solution. By measuring the amount of light that a sample absorbs, you can apply Beer's Law to calculate the concentration. This is done using a colorimeter or spectrophotometer.
The relationship between absorbance and concentration is linear, and it can be represented by a straight line with a y-intercept of zero. The slope of this line is equal to the product of the molar absorptivity (or extinction coefficient) and the path length.
To determine the concentration of a sample, you can use a spectrophotometer to find the absorbance (A) and path length (L). If the molar absorptivity (ε) is known, you can rearrange the equation to solve for concentration (c).
For example, let's say you have a sample with an absorbance of 0.70, a path length of 1 cm, and a molar absorptivity of 8400 M-1cm-1.
> 0.70 = (8400 M-1cm-1)(1 cm)(c)
Dividing both sides by [(8400 M-1 cm-1)(1 cm)], you get:
> c = 8.33 x 10-5 mol/L
So, the concentration of the sample is 8.33 x 10^-5 mol/L.
Fitts' Law: Designing Efficient Keyboards for Faster Typing
You may want to see also

The law is used to find the solution concentration and assess oxidation
Beer's Law, also known as the Beer-Lambert Law or
Miranda Rights: Do They Apply to Minors?
You may want to see also

The law is also used to measure the amount of bilirubin in blood samples
Beer's Law, also known as the Beer-Lambert Law, states that the absorption of light by a sample is directly proportional to its path length through the sample and the solution concentration. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. The law is expressed as:
> A = ε ℓ c
Where A is the measured absorbance, ε is the absorptivity or molar attenuation coefficient in M-1cm-1, ℓ is the optical path length in cm, and c is the concentration of the chemical species in mol/L or M.
The law is used in medicine to measure the amount of bilirubin in blood samples. Bilirubin is a yellow pigment in bile, which is a byproduct of broken-down old red blood cells. A bilirubin test measures bilirubin levels in the blood to determine whether they're in the normal range. High or low bilirubin levels can indicate that some part of the process of breaking down and clearing old red blood cells isn't working correctly.
A healthcare provider will usually take a blood sample from a vein in the patient's arm and send it to a lab for testing. The lab will measure the patient's bilirubin levels to determine whether they're within the normal range. Normal bilirubin levels for children and adults are between 0.2 and 1.3 mg/dL, while for newborns, normal levels can range from 1.0 to 12.0 mg/dL.
The Beer-Lambert law can be applied to the analysis of a mixture by spectrophotometry, without the need for extensive pre-processing of the sample. The spectrum of pure bilirubin is known, so the molar attenuation coefficient ε is known. Measurements of decadic attenuation coefficient μ10 are made at one wavelength λ that is nearly unique for bilirubin and at a second wavelength to correct for possible interferences. The amount concentration c is then given by:
> c = μ10(λ) / ε(λ)
This calculation allows technicians to use Beer's Law to measure the amount of bilirubin in blood samples.
Understanding Texas Lemon Law and Private Transactions
You may want to see also

Beer's Law is used in spectroscopy to describe the attenuation of solar radiation in the Earth's atmosphere
Beer's Law, or the Beer-Lambert Law, is a fundamental principle in spectroscopy that describes the attenuation of light as it passes through a substance. In the context of Earth and Planetary Sciences, it is used to explain the attenuation of solar radiation in the Earth's atmosphere.
The Beer-Lambert Law states that the absorption of light by a sample is directly proportional to its path length through the sample and the solution concentration. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. This law is expressed as:
> A = εbc
Where:
- A is the absorbance of the solution (no units)
- Ε is the molar absorptivity (units of l mol-1 cm-1)
- B is the path length of radiation through the absorbing medium (units of cm)
- C is the concentration (units of mol l-1)
The Beer-Lambert Law can be applied to the analysis of a mixture by spectrophotometry, without the need for extensive pre-processing of the sample. It is also used in atmospheric science to describe the attenuation of solar radiation as it travels through the Earth's atmosphere. In this case, the law takes into account both scattering and absorption of radiation.
The law has several applications beyond chemistry, including physics, medicine, and meteorology. It is a valuable tool for understanding and quantifying various natural phenomena.
Pascal's Law: Squeezing Toothpaste with Ease
You may want to see also

The law is used in physics to describe the attenuation of particle beams
The Beer-Lambert law, also known as Beer's law, is a linear relationship between the absorbance and concentration of a solution. It is used in physics to describe the attenuation of particle beams, such as neutron beams passing through matter.
The law states that the attenuation of light is directly proportional to the length of its path and the concentration of the solution. In other words, a solution absorbs more monochromatic light the further it passes through the sample or the more concentrated it is. This principle can be applied to particle beams, where the intensity of the beam decreases as it passes through a medium.
The Beer-Lambert law can be expressed mathematically as:
> A = ε ℓ c
Where:
- A is the measured absorbance
- Ε is the absorptivity or molar attenuation coefficient
- ℓ is the optical path length
- C is the concentration of the chemical species
The Beer-Lambert law is derived from the work of Pierre Bouguer, Johann Lambert, and August Beer. Bouguer's work in the early 18th century involved studying the intensity of starlight as it passed through the Earth's atmosphere. He discovered that light intensity decreased exponentially with the length travelled through the atmosphere. Lambert expressed this relationship mathematically in his 1760 work "Photometria". Beer built upon their work, observing that coloured solutions exhibited a similar attenuation relation. He found that the transmittance of a solution remained constant if the product of the path length and concentration was constant.
The Beer-Lambert law has applications beyond physics, including in chemistry, medicine, and meteorology. It is a valuable tool for understanding the behaviour of light and particle beams as they interact with various media.
Castle Law and Squatters: What's the Legal Verdict?
You may want to see also
Frequently asked questions
Beer's Law is an equation that relates the attenuation of light to the properties of a material. It states that a chemical's concentration is directly proportional to a solution's absorbance.
The Beer-Lambert Law equation is: A = εbc, where A is absorbance, ε is the molar absorptivity, b is the path length, and c is the concentration of the solution.
It is assumed that the absorbance is directly proportional to the sample's path length (the width of the cuvette) and its concentration.
Beer's Law relates only to concentration, whereas the Beer-Lambert Law relates absorbance to concentration and sample thickness.
You can use a colorimeter or spectrophotometer to measure the absorbance of a sample. Then, using the Beer-Lambert Law equation, rearrange the terms to solve for concentration.