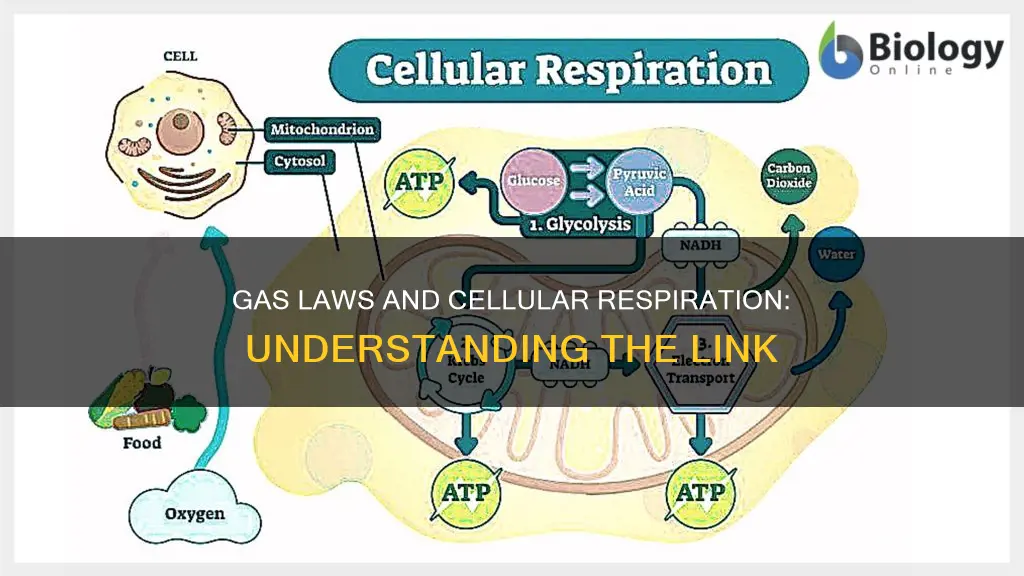
The ideal gas law, which describes the relationship between temperature, pressure, volume, and the number of molecules, is fundamental to understanding how a respirometer works. The modern form of the equation is: PV = nRT, where P is pressure, V is volume, n is the number of molecules, R is the gas constant, and T is temperature. This law is used to measure the rate of cellular respiration, which is the process of converting biochemical energy from nutrients into adenosine triphosphate (ATP) and releasing waste products. During cellular respiration, oxygen is consumed and carbon dioxide is released, and these volume changes can be measured using a respirometer. By introducing potassium hydroxide, which absorbs carbon dioxide, the respirometer can measure the consumption of oxygen gas by living cells.
| Characteristics | Values |
|---|---|
| State of an amount of gas | Determined by its pressure, volume, and temperature |
| Modern form of the ideal gas law equation | pV = nRT |
| p | Absolute pressure of the gas |
| V | Volume of the gas |
| n | Number of molecules of gas |
| R | Ideal or universal gas constant |
| T | Absolute temperature of the gas (in Ko) |
| If temperature and pressure are constant | Volume of the gas is directly proportional to the number of molecules of gas |
| If temperature and volume are constant | Pressure of the gas is in direct proportion to the number of molecules of gas present |
| If the number of gas molecules and the temperature are constant | Pressure is inversely proportional to the volume |
| If the temperature changes and the number of gas molecules are constant | Either pressure or volume (or both) will change in direct proportion to the temperature |
What You'll Learn

The ideal gas law and its role in understanding respirometers
The ideal gas law is fundamental to understanding how respirometers work. This law describes the relationship between the pressure, volume, and temperature of a gas, and is given by the equation:
PV = nRT
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the number of gas molecules
- R is the ideal or universal gas constant
- T is the absolute temperature of the gas in Kelvin
From this law, we can derive several principles that govern the behaviour of gases. For instance, if the temperature and pressure are constant, the volume of the gas is directly proportional to the number of gas molecules. Conversely, if the temperature and volume remain constant, the pressure of the gas is directly proportional to the number of molecules. These principles are essential for understanding gas behaviour in a respirometer, a device used to measure the volume of gas consumed or produced during cellular respiration.
During cellular respiration, cells undergo a series of metabolic reactions to convert biochemical energy from nutrients into adenosine triphosphate (ATP), releasing waste products such as carbon dioxide (CO2) and water (H2O). Respirometers are used to measure the volume of oxygen consumed or carbon dioxide produced during this process. By introducing an agent like potassium hydroxide (KOH) or sodium hydroxide (NaOH) into the respirometer, carbon dioxide can be removed, allowing for the measurement of oxygen consumption.
The ideal gas law is crucial for interpreting the data obtained from a respirometer. For example, if the temperature and volume inside the respirometer are kept constant, a decrease in pressure indicates a lower number of gas molecules, implying that oxygen has been consumed during cellular respiration. This understanding of the ideal gas law enables researchers to quantify respiration rates and gain insights into the metabolic processes occurring within cells.
In summary, the ideal gas law provides a foundation for comprehending the behaviour of gases within a respirometer. By manipulating variables such as temperature, volume, and pressure, scientists can measure gas volume changes and calculate respiration rates. This, in turn, enhances our understanding of cellular respiration and the underlying metabolic reactions that sustain life.
Compound Interest: Transform Your Personal Finance
You may want to see also

How respirometers measure respiration rates
Respirometers are devices used to measure the rate of respiration of living organisms by measuring their rate of exchange of oxygen and carbon dioxide. They can be used to investigate how factors such as age or chemicals affect the rate of respiration. Respirometers can be used to measure respiration on the level of a whole animal or plant, or on a cellular level.
A simple whole-plant respirometer designed to measure oxygen uptake or carbon dioxide release consists of a sealed container with the living specimen and a substance to absorb the carbon dioxide given off during respiration, such as soda lime pellets or cotton wads soaked with potassium hydroxide. The oxygen uptake is detected by manometry, typically using a U-tube manometer, which directly shows the pressure difference between the container and the atmosphere. As an organism takes in oxygen, it generates a proportionate quantity of carbon dioxide, but all the carbon dioxide is absorbed by the soda lime. Therefore, the entire drop in pressure in the chamber can be attributed to the drop in oxygen partial pressure in the container. The rate of change gives a reasonably accurate reading of the organism's respiration rate.
To control for changes in temperature or pressure, which can also affect the displacement of the manometric fluid, a second identical respirometer with a dead specimen may be set up. By subtracting the displacement of the second respirometer from the first, scientists can control for these factors.
Modern respirometers are available from various suppliers, depending on the intended use. For whole animal respirometers, suppliers include Sable Systems, Respirometer Systems and Applications, and Qubit Systems. For mitochondrial respirometers, suppliers include Oroboros Instruments, Hansatech Instruments, and YSI.
Respirometers are useful tools for understanding the ideal gas law in the context of cellular respiration. The ideal gas law describes the relationship between pressure, volume, temperature, and the number of moles of gas in a given volume. During cellular respiration, organisms combine oxygen with foodstuff molecules, converting the chemical energy into life-sustaining activities and discarding carbon dioxide and water as waste products. The ideal gas law can be applied to understand the behaviour of these gases during respiration.
HIPAA Law: Who Is Bound By It?
You may want to see also

The role of temperature in cellular respiration
The ideal gas law describes the relationship between pressure, volume, temperature, and the number of moles of a gas. In the context of cellular respiration, this law helps us understand the behaviour of respiratory gases under different conditions.
Temperature plays a significant role in cellular respiration, which is the process by which organisms combine oxygen with food molecules to produce energy. This process can be broken down into three main metabolic stages: glycolysis, the tricarboxylic acid (TCA) cycle, and oxidative phosphorylation.
As temperatures rise, the motion of particles increases, and this increased speed leads to more frequent collisions between substrate (reactant) molecules and enzymes. The rate of cellular respiration is dependent on these enzyme interactions, so an increase in temperature results in a faster reaction.
However, there is an upper limit to this relationship. If the temperature rises too high, the enzymes responsible for cellular respiration will denature, causing the reaction to slow down and eventually stop as more enzymes are affected.
Conversely, a decrease in temperature will slow down the rate of reaction as the molecular energy of the reactants decreases, resulting in fewer collisions. In plants, a decrease in temperature will eventually lead to the freezing of tissues.
Therefore, temperature plays a critical role in the rate of cellular respiration, with an optimal range that is specific to the organism.
Consulting and Public Law 86-272: What's the Verdict?
You may want to see also

The difference between germinating and dormant seeds in cellular respiration
The ideal gas law describes the relationship between the pressure, volume, and temperature of a given mass of ideal gas. This law is a combination of Boyle's law, Charles' law, and Gay-Lussac's law, and it is used to understand the behaviour of gases in various conditions.
Now, let's discuss the difference between germinating and dormant seeds in cellular respiration:
During the dormant period, seeds exhibit low cellular respiration rates, just enough to maintain their nutrient supply within the endosperm layer. This layer is formed during double fertilization in flowering plants and provides the necessary nutrients for the seed during dormancy. The seed's primary goal during this stage is to preserve its energy reserves while waiting for the optimal environmental conditions for growth.
As soon as germination begins, the seed's cellular respiration rates increase significantly. This increase in cellular respiration is essential to provide the energy required for initial plant growth. The seed breaks open, and the initial root and stem structures are formed. The seed's oxygen consumption increases during this active growth phase.
The process of cellular respiration in germinating seeds involves three stages: glycolysis, the Krebs Cycle, and the Electron Transport Chain. During glycolysis, glucose molecules are used to produce energy in the form of ATP (adenosine triphosphate) molecules, along with other chemical materials. The Krebs Cycle uses the products from glycolysis to generate more energy and transform leftover chemicals. Finally, the Electron Transport Chain combines the energy from the Krebs Cycle with oxygen to create even more ATP molecules.
In summary, the key difference between germinating and dormant seeds in cellular respiration lies in the rate of cellular respiration. Dormant seeds maintain low respiration rates to conserve energy, while germinating seeds exhibit a significant increase in respiration rates to support the energy demands of growth and development.
International Waters: Navigating Complex Legal Waters
You may want to see also

The application of the ideal gas law in cellular respiration
The ideal gas law, which states that the product of the pressure and volume of a gas is proportional to the number of molecules of the gas and the temperature, is fundamental to understanding how a respirometer works. The pressure, volume, and temperature of a gas are determined by its state.
The modern form of the ideal gas law equation is:
[math]pV = nRT\,[/math]
Where:
- P is the absolute pressure of the gas
- V is the volume of the gas
- N is the number of molecules of gas
- R is the ideal, or universal, gas constant
- T is the absolute temperature of the gas (in Kelvin)
In the context of cellular respiration, the ideal gas law can be applied to understand the relationship between temperature, volume, and pressure. For instance, if the temperature and volume remain constant, the pressure of the gas is directly proportional to the number of gas molecules. Conversely, if the number of gas molecules and the temperature remain constant, the pressure is inversely proportional to the volume.
During cellular respiration, the volume of two gases changes: oxygen is consumed by the respiring cells, and carbon dioxide is released. To measure the rate of cellular respiration, scientists use a respirometer, which measures changes in gas volume. To account for the simultaneous changes in gas volumes, potassium hydroxide (KOH) is introduced into the device. KOH absorbs carbon dioxide according to the equation:
CO2 + 2KOH → K2CO3 + H2O
Potassium carbonate (K2CO3) is a solid precipitate, so any CO2 produced is converted from a gas to a solid and is no longer affected by gas laws. This allows the respirometer to solely measure the consumption of oxygen gas by living cells.
In a practical application of the ideal gas law, a lab experiment may involve comparing the relative volume of oxygen consumed by germinating and non-germinating pea seeds at different temperatures. By submerging respirometers containing the seeds in water baths of different temperatures, the effect of temperature on the rate of oxygen consumption can be observed. The respirometers will be sealed to ensure no gas escapes, and the water level in the tubing attached to the respirometer will indicate the change in gas volume.
In summary, the ideal gas law is essential for understanding the workings of a respirometer, a device used to measure gas volume changes during cellular respiration. By introducing KOH to absorb carbon dioxide, the respirometer can specifically measure oxygen consumption. This allows scientists to study the impact of factors like temperature and seed germination on the rate of cellular respiration.
Lemon Law: Out-of-State Buyers' Rights Explained
You may want to see also







